Abstract
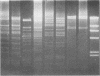
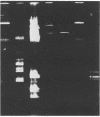
Bacterial strains isolated from deep-sea amphipods were identified, classified, and screened for plasmid content. Plasmids were common, with 11 of 16 isolates carrying one or more plasmids; these ranged in size from 2.9 to 63 megadaltons. Several of the strains demonstrated distinctly different phenotypic traits yet contained plasmids of the same molecular weight. Results of agarose gel electrophoresis, DNA hybridization, and restriction analysis indicate that the plasmids detected in these deep-sea isolates are identical, suggesting that transmission may occur in the deep-sea environment and that plasmids are common in some deep-sea habitats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altherr M. R., Kasweck K. L. In situ studies with membrane diffusion chambers of antibiotic resistance transfer in Escherichia coli. Appl Environ Microbiol. 1982 Oct;44(4):838–843. doi: 10.1128/aem.44.4.838-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell J. L., Lewis D. A., Reeves D. S. A rapid single colony lysate method for the selective visualization of plasmids in Enterobacteriaceae, including Serratia marcescens. J Antimicrob Chemother. 1981 Dec;8(6):481–485. doi: 10.1093/jac/8.6.481. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschek H. P., Klacik M. A. Role of DNase in recovery of plasmid DNA from Clostridium perfringens. Appl Environ Microbiol. 1984 Jul;48(1):178–181. doi: 10.1128/aem.48.1.178-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Warren G. J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E. M., Chace N. M., London S. B., London J. Transfer of plasmid-mediated antibiotic resistance from streptococci to lactobacilli. J Bacteriol. 1979 Jan;137(1):614–619. doi: 10.1128/jb.137.1.614-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Colwell R. R. Isolation of cryptic plasmid deoxyribonucleic acid from Kanagawa-positive strains of Vibrio parahaemolyticus. Infect Immun. 1977 Apr;16(1):328–334. doi: 10.1128/iai.16.1.328-334.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada H. S., Sizemore R. K. Incidence of Plasmids in Marine Vibrio spp. Isolated from an Oil Field in the Northwestern Gulf of Mexico. Appl Environ Microbiol. 1981 Jan;41(1):199–202. doi: 10.1128/aem.41.1.199-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H., Sullivan C. W., Shizuya H. Bacterial plasmids in antarctic natural microbial assemblages. Appl Environ Microbiol. 1984 Sep;48(3):515–518. doi: 10.1128/aem.48.3.515-518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mach P. A., Grimes D. J. R-plasmid transfer in a wastewater treatment plant. Appl Environ Microbiol. 1982 Dec;44(6):1395–1403. doi: 10.1128/aem.44.6.1395-1403.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Nelson J. D., Jr, Justice A., Colwell R. R. Incidence of Salmonella spp., Clostridium botulinum, and Vibrio parahaemolyticus in an estuary. Appl Environ Microbiol. 1976 May;31(5):723–730. doi: 10.1128/aem.31.5.723-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Clewell D. B., Glatzer L. Conjugative transfer of R-plasmids from Streptococcus faecalis to Staphylococcus aureus. Antimicrob Agents Chemother. 1982 Aug;22(2):204–207. doi: 10.1128/aac.22.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Cabelli V. J. R-plasmid transfer frequencies from environmental isolates of Escherichia coli to laboratory and fecal strains. Appl Environ Microbiol. 1980 Oct;40(4):756–764. doi: 10.1128/aem.40.4.756-764.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore R. K., Colwell R. R. Plasmids carried by antibiotic-resistant marine bacteria. Antimicrob Agents Chemother. 1977 Sep;12(3):373–382. doi: 10.1128/aac.12.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. The effect on virulence of transferring R factors to Salmonella typhimurium in vivo. J Med Microbiol. 1972 Nov;5(4):451–458. doi: 10.1099/00222615-5-4-451. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timmis K., Winkler U. Isolation of covalently closed circular deoxyribonucleic acid from bacteria which produce exocellular nuclease. J Bacteriol. 1973 Jan;113(1):508–509. doi: 10.1128/jb.113.1.508-509.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Yeggy J. P., Markovetz A. J. Role of nucleases in the isolation of plasmid deoxyribonucleic acid from Pseudomonas cepacia 4G9. J Bacteriol. 1980 Aug;143(2):1057–1059. doi: 10.1128/jb.143.2.1057-1059.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]