Abstract
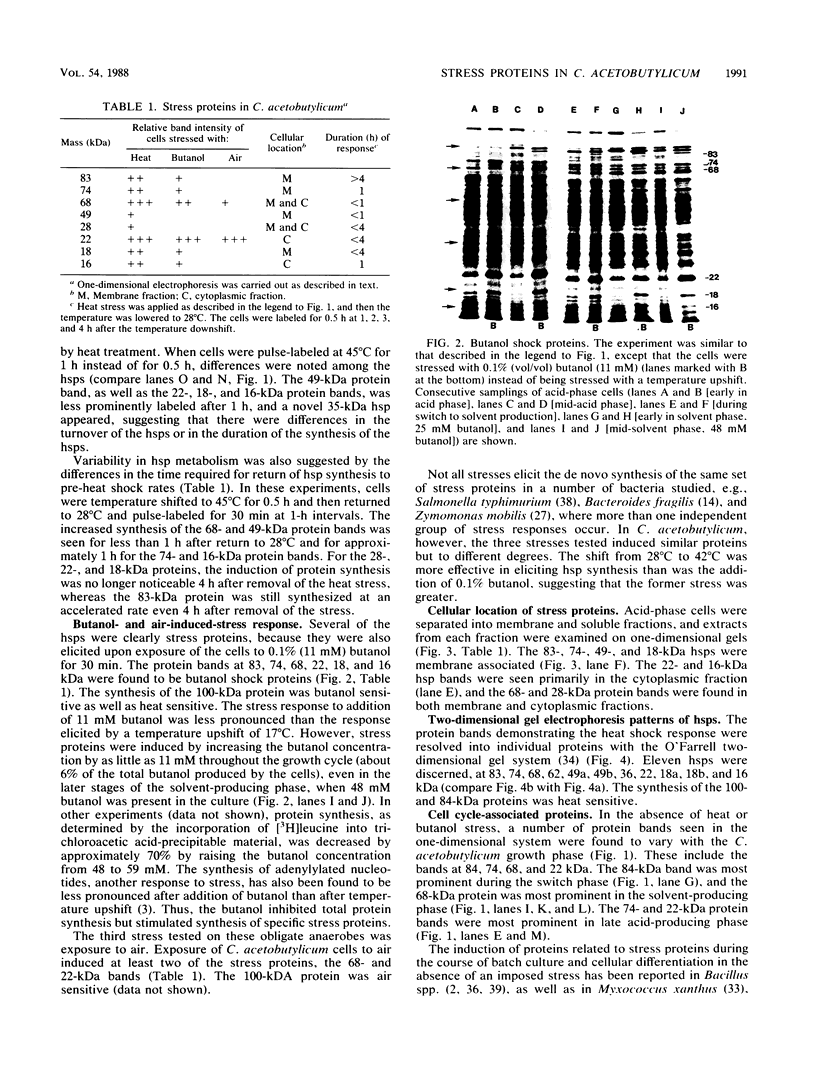
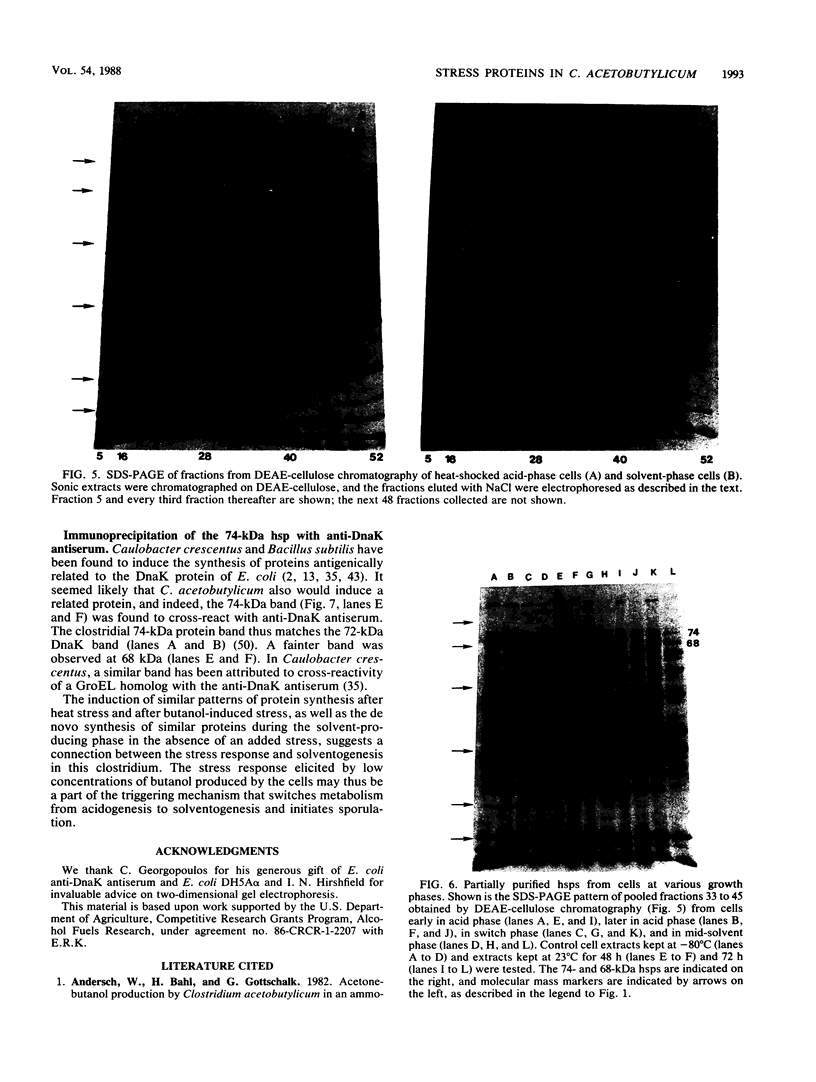
The response of Clostridium acetobutylicum ATCC 4259 to the stresses produced by a temperature upshift from 28°C to 45°C and by exposure of the organisms to 0.1% n-butanol or to air was examined by analysis of pulse-labeled proteins. The stress response was the induction of the synthesis of a number of proteins, some of which were elicited by the three forms of stress. Eleven heat shock proteins were identified by two-dimensional electrophoresis, as were two proteins whose synthesis was heat sensitive. In the absence of applied stress, the synthesis of four proteins was found to be associated with the growth phase in batch culture; three of these proteins had a higher rate of de novo synthesis when the cells entered the solvent production phase. One of the stress-induced proteins, hsp74, was partially purified an found to be immunologically related to Escherichia coli heat shock protein Dnak. The similarities of the proteins induced at the onset of solventogenesis and by stress suggest a relationship between the two processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodimos I. A., Kashket E. R., Rapaport E. Metabolism of adenylylated nucleotides in Clostridium acetobutylicum. J Bacteriol. 1988 May;170(5):2301–2305. doi: 10.1128/jb.170.5.2301-2305.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M. C., Silva A. M., Gomes S. L., Maia J. C., Juliani M. H. Differential expression of heat-shock proteins and spontaneous synthesis of HSP70 during the life cycle of Blastocladiella emersonii. Eur J Biochem. 1987 Feb 16;163(1):211–220. doi: 10.1111/j.1432-1033.1987.tb10757.x. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Daniels C. J., McKee A. H., Doolittle W. F. Archaebacterial heat-shock proteins. EMBO J. 1984 Apr;3(4):745–749. doi: 10.1002/j.1460-2075.1984.tb01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. L., Juliani M. H., Maia J. C., Silva A. M. Heat shock protein synthesis during development in Caulobacter crescentus. J Bacteriol. 1986 Nov;168(2):923–930. doi: 10.1128/jb.168.2.923-930.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. J., Strydom E., Woods D. R. Heat shock stress in Bacteroides fragilis. Arch Microbiol. 1985 Sep;142(4):362–364. doi: 10.1007/BF00491904. [DOI] [PubMed] [Google Scholar]
- Hutkins R. W., Kashket E. R. Phosphotransferase Activity in Clostridium acetobutylicum from Acidogenic and Solventogenic Phases of Growth. Appl Environ Microbiol. 1986 May;51(5):1121–1123. doi: 10.1128/aem.51.5.1121-1123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. L., Kirk M. M. Heat shock elicits production of sexual inducer in Volvox. Science. 1986 Jan 3;231(4733):51–54. doi: 10.1126/science.3941891. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Michel G. P., Starka J. Effect of ethanol and heat stresses on the protein pattern of Zymomonas mobilis. J Bacteriol. 1986 Mar;165(3):1040–1042. doi: 10.1128/jb.165.3.1040-1042.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S., Buzin C. H. Self-degradation of heat shock proteins. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4969–4973. doi: 10.1073/pnas.82.15.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Killeen K. P. Heat shock proteins of vegetative and fruiting Myxococcus xanthus cells. J Bacteriol. 1986 Dec;168(3):1100–1106. doi: 10.1128/jb.168.3.1100-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reuter S. H., Shapiro L. Asymmetric segregation of heat-shock proteins upon cell division in Caulobacter crescentus. J Mol Biol. 1987 Apr 20;194(4):653–662. doi: 10.1016/0022-2836(87)90242-7. [DOI] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streips U. N., Polio F. W. Heat shock proteins in bacilli. J Bacteriol. 1985 Apr;162(1):434–437. doi: 10.1128/jb.162.1.434-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglicht D., Padan E., Oppenheim A. B., Schuldiner S. An alkaline shift induces the heat shock response in Escherichia coli. J Bacteriol. 1987 Feb;169(2):885–887. doi: 10.1128/jb.169.2.885-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Hubbard T. J., Travers A. A., Ellar D. J. Heat-shock proteins during growth and sporulation of Bacillus subtilis. FEBS Lett. 1985 Sep 2;188(2):209–214. doi: 10.1016/0014-5793(85)80373-2. [DOI] [PubMed] [Google Scholar]
- Tsuchido T., VanBogelen R. A., Neidhardt F. C. Heat shock response in Escherichia coli influences cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6959–6963. doi: 10.1073/pnas.83.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Giannini S. H., Cantor C. R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985 Jun 21;228(4706):1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]
- Zylicz M., Georgopoulos C. Purification and properties of the Escherichia coli dnaK replication protein. J Biol Chem. 1984 Jul 25;259(14):8820–8825. [PubMed] [Google Scholar]