Abstract
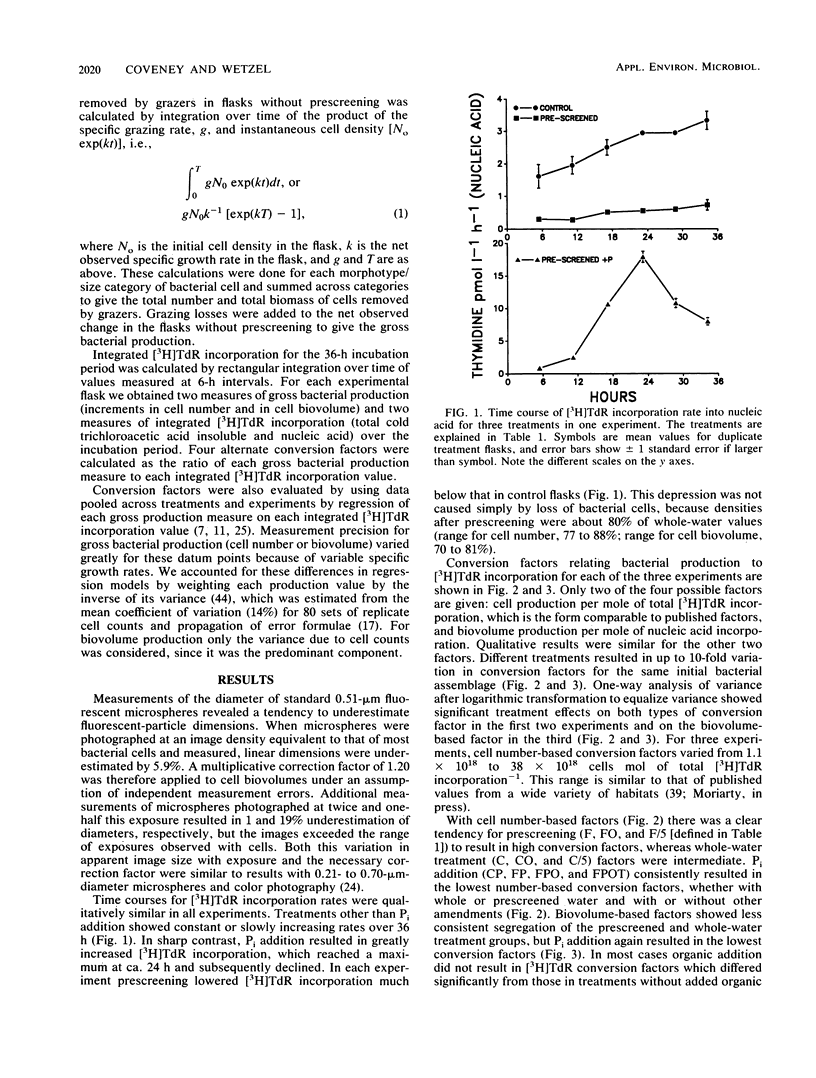
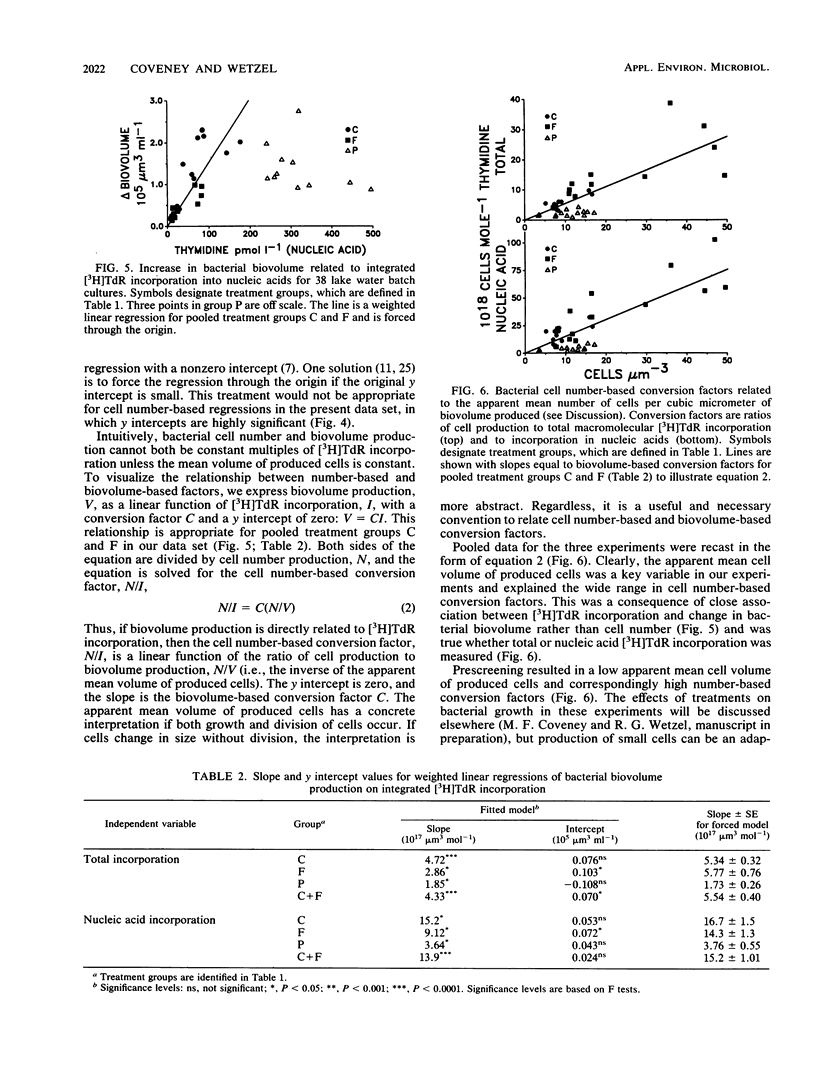
The relationship between bacterial growth and incorporation of [methyl-3H]thymidine in oligotrophic lake water cultures was investigated. Prescreening, dilution, and addition of organic and inorganic nutrients were treatments used to prevent bacterivory and stimulate bacterial growth. Growth in unmanipulated samples was estimated through separate measurements of grazing losses. Both bacterial number and biovolume growth responses were measured, and incorporation of [3H]thymidine in both total macromolecules and nucleic acids was assayed. The treatments had significant effects on conversion factors used to relate thymidine incorporation to bacterial growth. Cell number-based factors ranged from 1.1 × 1018 to 38 × 1018 cells mol of total thymidine incorporation−1 and varied with treatment up to 10-fold for the same initial bacterial assemblage. In contrast, cell biovolume-based conversion factors were similar for two treatment groups across a 16-fold range of [3H]thymidine incorporation rates: 5.54 × 1017 μm3 mol of total thymidine incorporation−1 and 15.2 × 1017 μm3 mol of nucleic acid incorporation−1. Much of the variation in cell number-based conversion factors was related to changes in apparent mean cell volume of produced bacteria. Phosphorus addition stimulated [3H]thymidine incorporation more than it increased bacterial growth, which resulted in low conversion factors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. T. Further Verification of the Isotope Dilution Approach for Estimating the Degree of Participation of [H]thymidine in DNA Synthesis in Studies of Aquatic Bacterial Production. Appl Environ Microbiol. 1986 Nov;52(5):1212–1214. doi: 10.1128/aem.52.5.1212-1214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern L. Autoradiographic Studies of [methyl-H]Thymidine Incorporation in a Cyanobacterium (Microcystis wesenbergii)-Bacterium Association and in Selected Algae and Bacteria. Appl Environ Microbiol. 1985 Jan;49(1):232–233. doi: 10.1128/aem.49.1.232-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Bacterial growth rate in the sea: direct analysis by thymidine autoradiography. Science. 1967 Jan 6;155(3758):81–83. doi: 10.1126/science.155.3758.81. [DOI] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- Goodwin B. C. Synchronization of Escherichia coli in a chemostat by periodic phosphate feeding. Eur J Biochem. 1969 Oct;10(3):511–514. doi: 10.1111/j.1432-1033.1969.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., K'nees E., Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985 Mar;49(3):599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Fuhrman J. A. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton. Appl Environ Microbiol. 1987 Jun;53(6):1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Seasonal bacterial production in a dimictic lake as measured by increases in cell numbers and thymidine incorporation. Appl Environ Microbiol. 1985 Mar;49(3):492–500. doi: 10.1128/aem.49.3.492-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Annual cycle of bacterial secondary production in five aquatic habitats of the okefenokee swamp ecosystem. Appl Environ Microbiol. 1985 Mar;49(3):650–655. doi: 10.1128/aem.49.3.650-655.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts R. D., Wicks R. J., Sephton L. M. Spatial and Temporal Variations in Bacterial Macromolecule Labeling with [methyl-H]Thymidine in a Hypertrophic Lake. Appl Environ Microbiol. 1986 Dec;52(6):1368–1373. doi: 10.1128/aem.52.6.1368-1373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Ohkido S. Further studies on thymidine kinase: distribution pattern of the enzyme in bacteria. J Gen Microbiol. 1985 Nov;131(11):3091–3098. doi: 10.1099/00221287-131-11-3091. [DOI] [PubMed] [Google Scholar]
- Sanders R. W., Porter K. G. Use of metabolic inhibitors to estimate protozooplankton grazing and bacterial production in a monomictic eutrophic lake with an anaerobic hypolimnion. Appl Environ Microbiol. 1986 Jul;52(1):101–107. doi: 10.1128/aem.52.1.101-107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P., Billen G., Rego J. V. Rate of bacterial mortality in aquatic environments. Appl Environ Microbiol. 1985 Jun;49(6):1448–1454. doi: 10.1128/aem.49.6.1448-1454.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais P., Martinez J., Billen G., Vives-Rego J. Determining [H]Thymidine Incorporation into Bacterioplankton DNA: Improvement of the Method by DNase Treatment. Appl Environ Microbiol. 1987 Aug;53(8):1977–1979. doi: 10.1128/aem.53.8.1977-1979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T., Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. T., Pace M. L. Validity of eucaryote inhibitors for assessing production and grazing mortality of marine bacterioplankton. Appl Environ Microbiol. 1987 Jan;53(1):119–128. doi: 10.1128/aem.53.1.119-128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine S. C., Mills A. L. Inadequacy of the eucaryote inhibitor cycloheximide in studies of protozoan grazing on bacteria at the freshwater-sediment interface. Appl Environ Microbiol. 1987 Aug;53(8):1969–1972. doi: 10.1128/aem.53.8.1969-1972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn C. D., Karl D. M. Laboratory calibrations of the [h]adenine technique for measuring rates of RNA and DNA synthesis in marine microorganisms. Appl Environ Microbiol. 1984 Apr;47(4):835–842. doi: 10.1128/aem.47.4.835-842.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


