Abstract
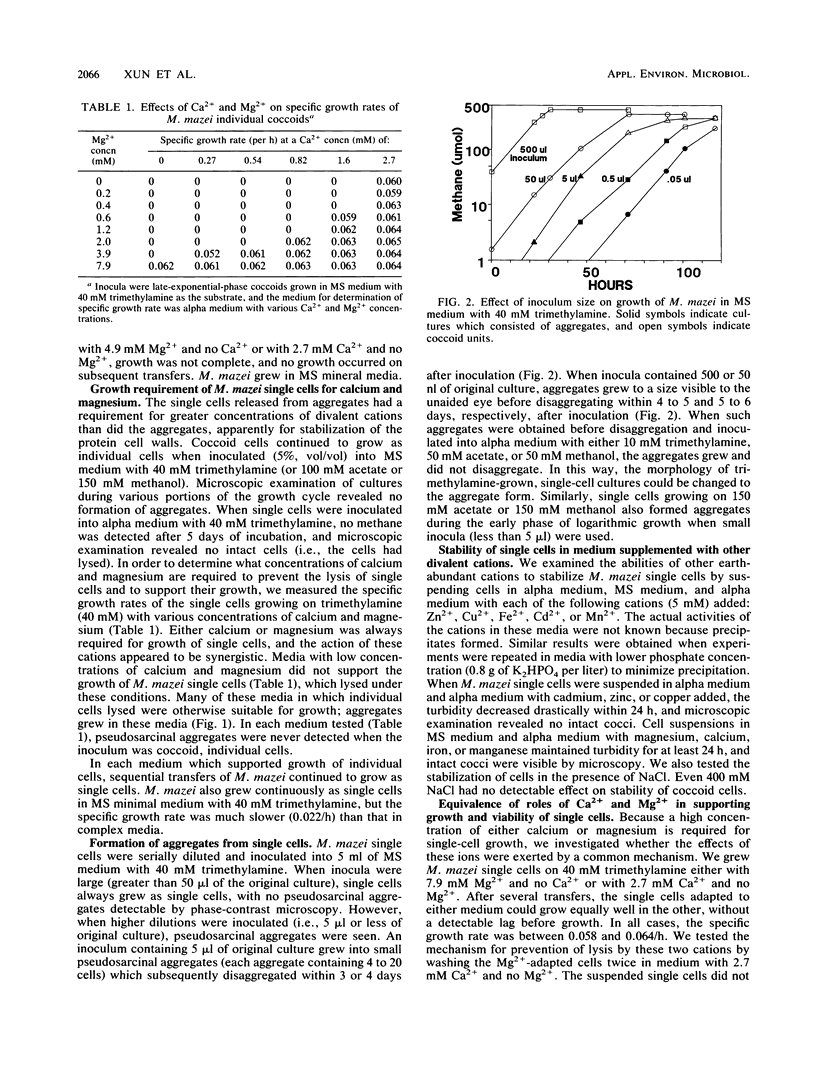
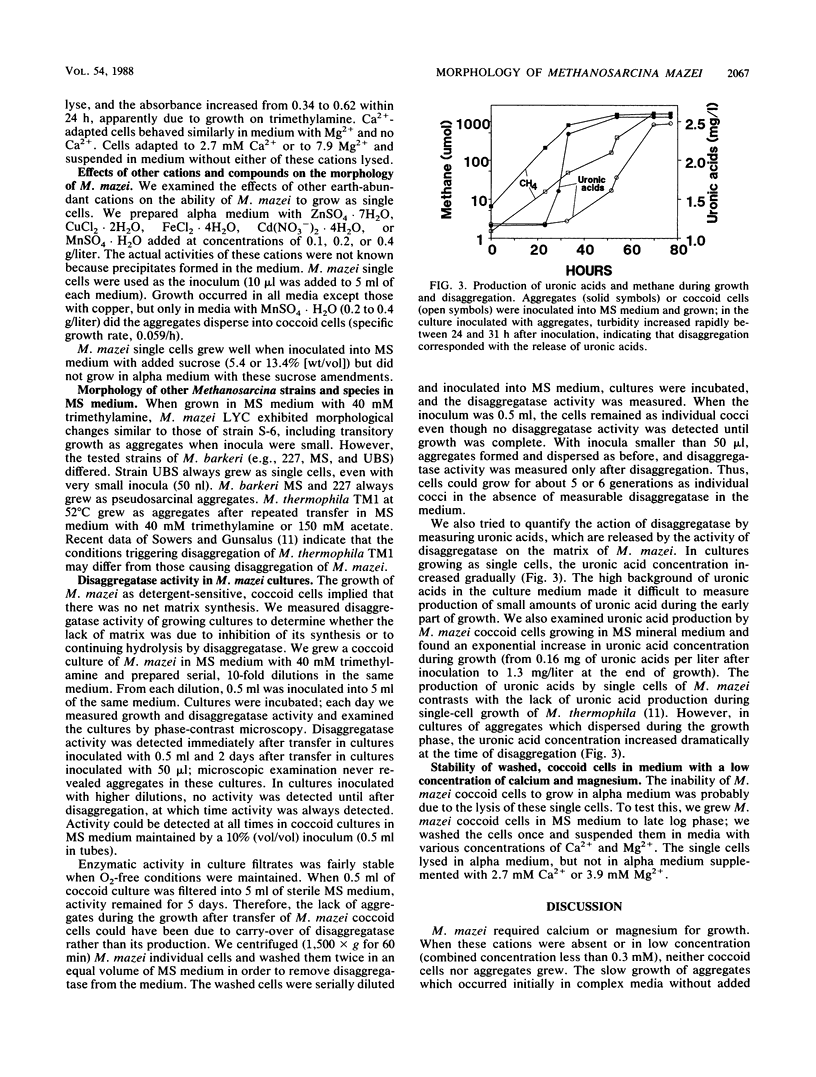
The morphology of Methanosarcina mazei was controlled by magnesium, calcium, and substrate concentrations and by inoculum size; these factors allowed manipulation of the morphology and interconversions between pseudosarcinal aggregates and individual, coccoid cells. M. mazei grew as aggregates in medium with a low concentration of catabolic substrate (either 50 mM acetate, 50 mM methanol, or 10 mM trimethylamine) unless Ca2+ and Mg2+ concentrations were high. Growth in medium high in Ca2+, Mg2+, and substrate (i.e., 150 mM acetate, 150 mM methanol, or 40 mM trimethylamine) converted pseudosarcinal aggregates to individual cocci. In such media, aggregates separated into individual cells which continued to grow exclusively as single cells during subsequent transfers. Conversion of single cells back to aggregates was complicated, because conditions which supported the aggregated morphology (e.g., low calcium or magnesium concentration) caused lysis of coccoid inocula. We recovered aggregates from coccoid cells by inoculating serial dilutions into medium high in calcium and magnesium. Cells from very dilute inocula grew into aggregates which disaggregated on continued incubation. However, timely transfer of the aggregates to medium low in calcium, magnesium, and catabolic substrates allowed continued growth as aggregates. We demonstrated the activity of the enzyme (disaggregatase) which caused the dispersion of aggregates into individual cells; disaggregatase was produced not only during disaggregation but also in growing cultures of single cells. Uronic acids, the monomeric constituents of the Methanosarcina matrix, were also produced during disaggregation and during growth as coccoids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baresi L., Mah R. A., Ward D. M., Kaplan I. R. Methanogenesis from acetate: enrichment studies. Appl Environ Microbiol. 1978 Jul;36(1):186–197. doi: 10.1128/aem.36.1.186-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Mah R. A. Effects of Calcium, Magnesium, pH, and Extent of Growth on the Morphology of Methanosarcina mazei S-6. Appl Environ Microbiol. 1987 Jul;53(7):1699–1700. doi: 10.1128/aem.53.7.1699-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Xun L. Effects of pH, Temperature, and Nutrients on Propionate Degradation by a Methanogenic Enrichment Culture. Appl Environ Microbiol. 1987 Jul;53(7):1589–1592. doi: 10.1128/aem.53.7.1589-1592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Harris J. E. Spontaneous Disaggregation of Methanosarcina mazei S-6 and Its Use in the Development of Genetic Techniques for Methanosarcina spp. Appl Environ Microbiol. 1987 Oct;53(10):2500–2504. doi: 10.1128/aem.53.10.2500-2504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Boone D. R., Sleat R., Mah R. A. Methanosarcina mazei LYC, a New Methanogenic Isolate Which Produces a Disaggregating Enzyme. Appl Environ Microbiol. 1985 Mar;49(3):608–613. doi: 10.1128/aem.49.3.608-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W. Life Cycles in the Methanogenic Archaebacterium Methanosarcina mazei. Appl Environ Microbiol. 1986 Jul;52(1):17–27. doi: 10.1128/aem.52.1.17-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Gunsalus R. P. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J Bacteriol. 1988 Feb;170(2):998–1002. doi: 10.1128/jb.170.2.998-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


