Abstract
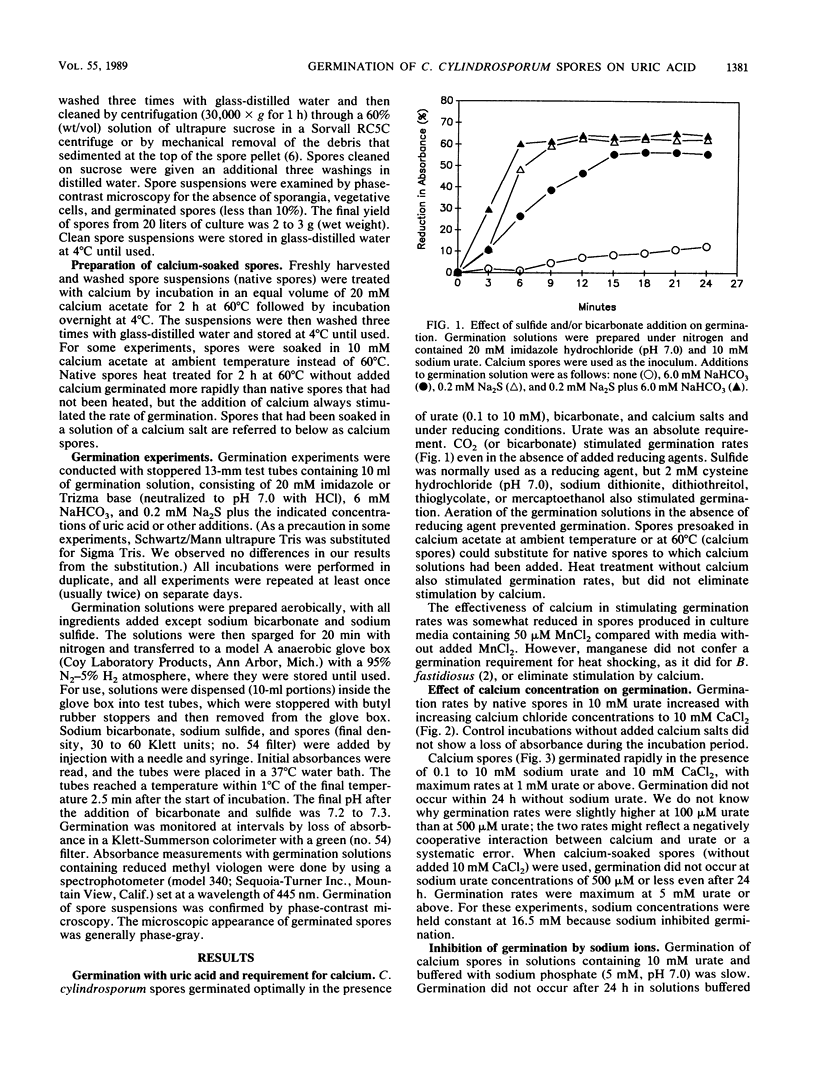
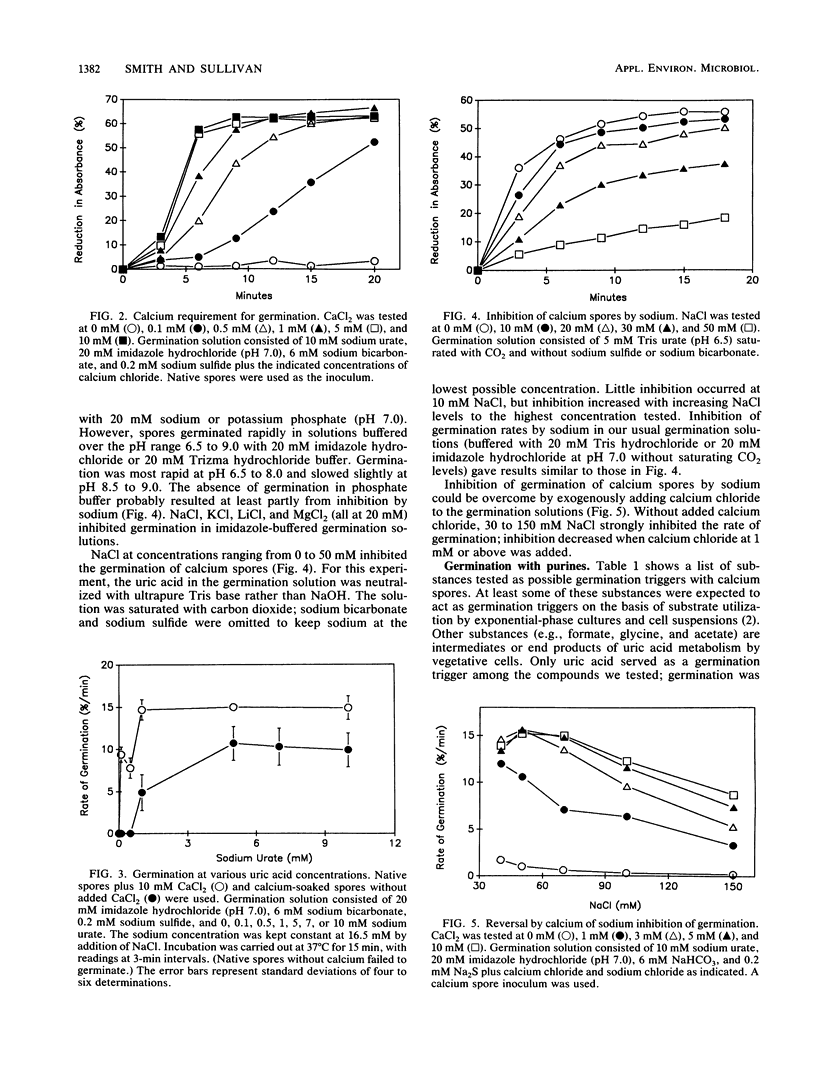
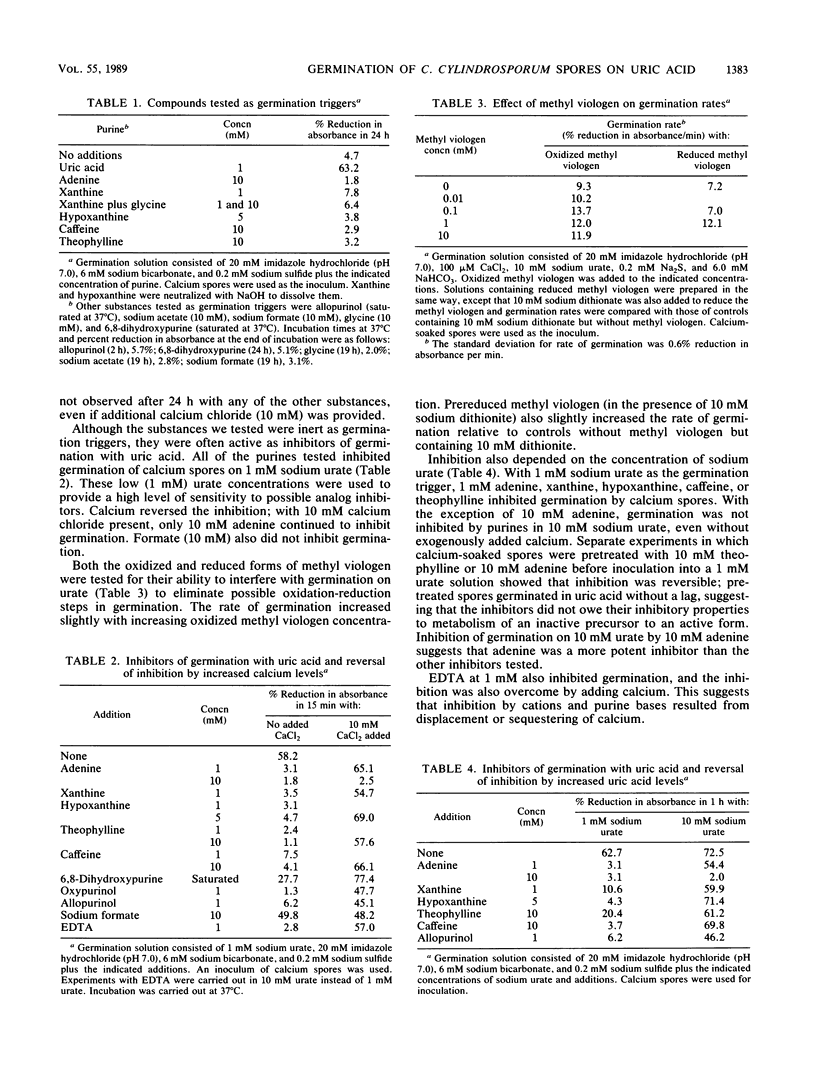
Clostridium cylindrosporum spores germinated rapidly under reducing conditions when bicarbonate, uric acid, and calcium were present. Germination rates on 10 mM urate increased with increasing Ca2+ (maximum rate at 5 mM Ca2+ or greater). Germination rates on urate (limiting Ca2+) increased with increasing urate concentrations to 10 mM urate. At 10 mM Ca2+, germination rates reached a maximum at 1 mM urate and remained constant thereafter. Cations (Na+, K+, Li+, and Mg2+), purines, purine analogs, and EDTA inhibited germination at limiting calcium concentrations but not (except for 10 mM adenine) at 10 mM Ca2+. Methyl viologen or formate did not inhibit germination. Germination was not observed in solutions containing xanthine, hypoxanthine, caffeine, theophylline, 6,8-dihydroxypurine, adenine, allopurinol, formate, glycine, or acetate, even though some of the purines are growth substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Slepecky R. A. Inducement of a heat-shock requirement for germination and production of increased heat resistance in Bacillus fastidiosus spores by manganous ions. J Bacteriol. 1973 Apr;114(1):137–143. doi: 10.1128/jb.114.1.137-143.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Beck J. V. Clostridium acidi-uridi and Clostridium cylindrosporum, Organisms Fermenting Uric Acid and Some Other Purines. J Bacteriol. 1942 Mar;43(3):291–304. doi: 10.1128/jb.43.3.291-304.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARNEY J., FISHER W. P., HEGARTY C. P. Managanese as an essential element for sporulation in the genus Bacillus. J Bacteriol. 1951 Aug;62(2):145–148. doi: 10.1128/jb.62.2.145-148.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Imagawa M., Takubo Y., Nishikawa J., Nishihara T., Kondo M. Germination of the decoated spores of Bacillus megaterium. Microbiol Immunol. 1985;29(12):1139–1149. doi: 10.1111/j.1348-0421.1985.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Tani K., Imagawa M., Nishihara T., Kondo M. Germinability of coat-lacking spores of Bacillus megaterium. Biochem Biophys Res Commun. 1985 Apr 30;128(2):728–732. doi: 10.1016/0006-291x(85)90107-x. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. GASEOUS HYDROCARBONS AND THE GERMINATION OF BACTERIAL SPORES. Proc Natl Acad Sci U S A. 1965 Jan;53:31–38. doi: 10.1073/pnas.53.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Ionic germination of spores of Bacillus megaterium QM B 1551. Arch Mikrobiol. 1962;43:183–200. doi: 10.1007/BF00406435. [DOI] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Influence of exchangeable ions on germinability of bacterial spores. J Bacteriol. 1966 Apr;91(4):1582–1588. doi: 10.1128/jb.91.4.1582-1588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Quantitative aspects of exchangeable calcium in spores of Bacillus megaterium. J Bacteriol. 1966 Apr;91(4):1589–1593. doi: 10.1128/jb.91.4.1589-1593.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. B., Levinson H. S. Changes in spores of Bacillus megaterium treated with thioglycolate at a low pH and restoration of germinability and heat resistance by cations. J Bacteriol. 1967 Mar;93(3):1017–1022. doi: 10.1128/jb.93.3.1017-1022.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks L. E., Smith M. R. Sporulation of Clostridium cylindrosporum on a Defined, Low-Manganese Medium. Appl Environ Microbiol. 1987 Jul;53(7):1696–1698. doi: 10.1128/aem.53.7.1696-1698.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas J. A., Johnstone K., Ellar D. J. Role of uricase in the triggering of germination of Bacillus fastidiosus spores. Biochem J. 1985 Jul 1;229(1):241–249. doi: 10.1042/bj2290241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer-Ullrich H., Wagner R., Dürre P., Andreesen J. R. Comparative studies on physiology and taxonomy of obligately purinolytic clostridia. Arch Microbiol. 1984 Aug;138(4):345–353. doi: 10.1007/BF00410902. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Lequerica J. L. Methanosarcina mutant unable to produce methane or assimilate carbon from acetate. J Bacteriol. 1985 Nov;164(2):618–625. doi: 10.1128/jb.164.2.618-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Andreesen J. R. Differentiation between Clostridium acidiurici and Clostridium cylindrosporum on the basis of specific metal requirements for formate dehydrogenase formation. Arch Microbiol. 1977 Sep 28;114(3):219–224. doi: 10.1007/BF00446865. [DOI] [PubMed] [Google Scholar]


