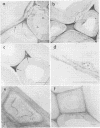
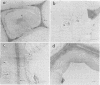
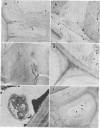
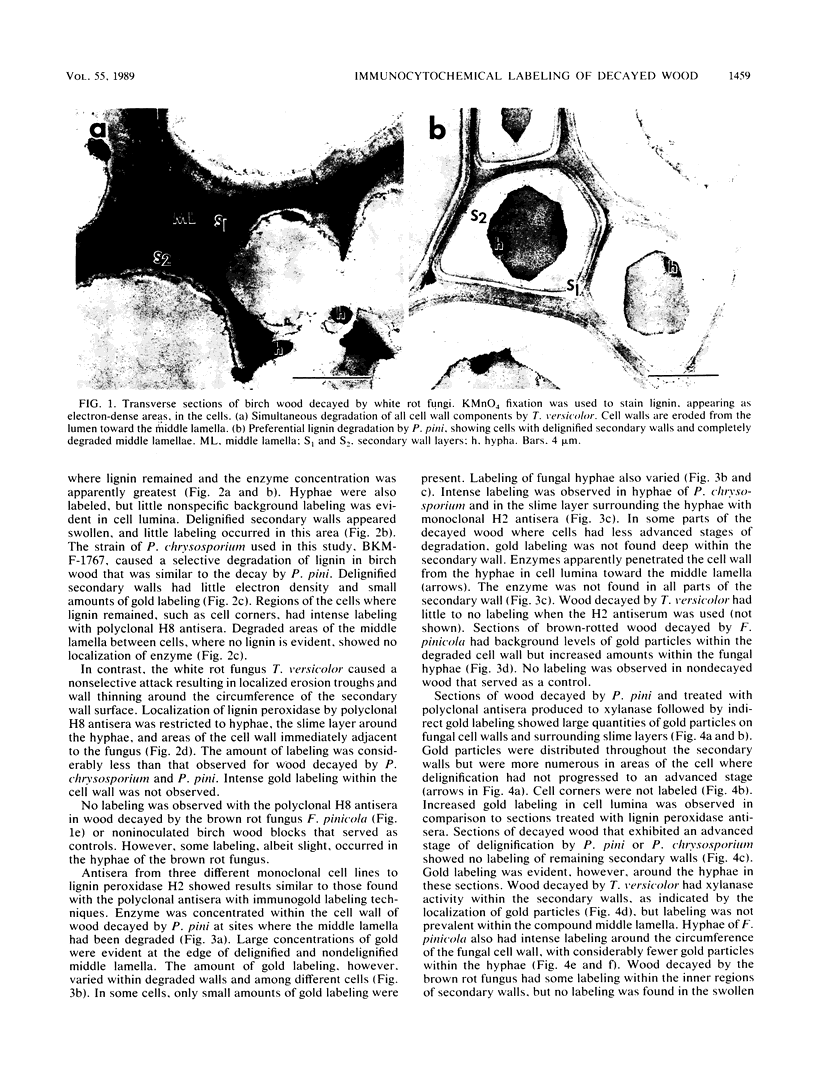
Abstract
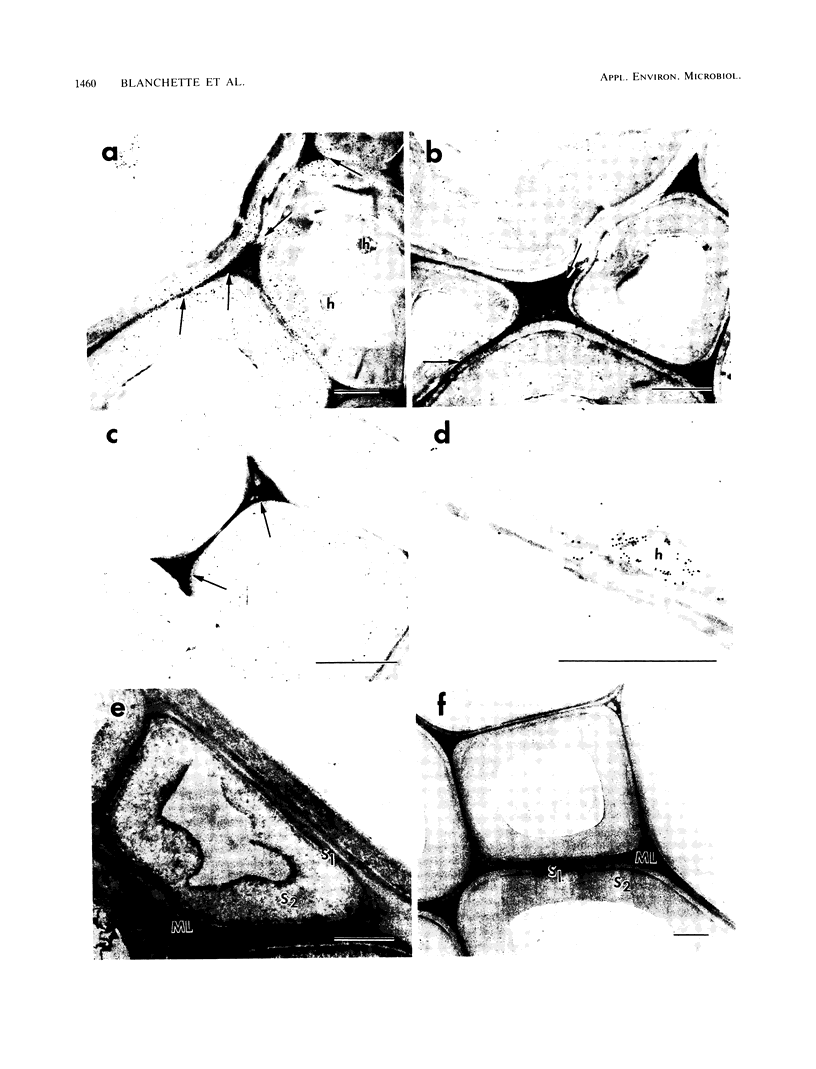
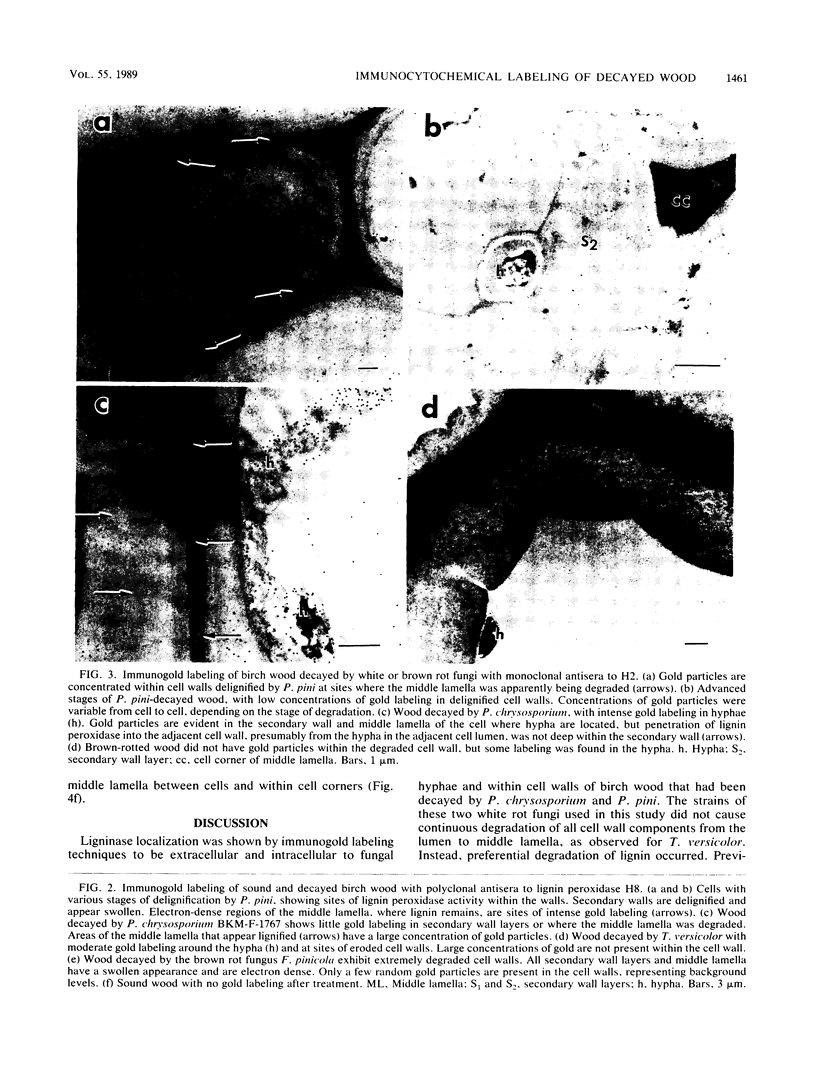
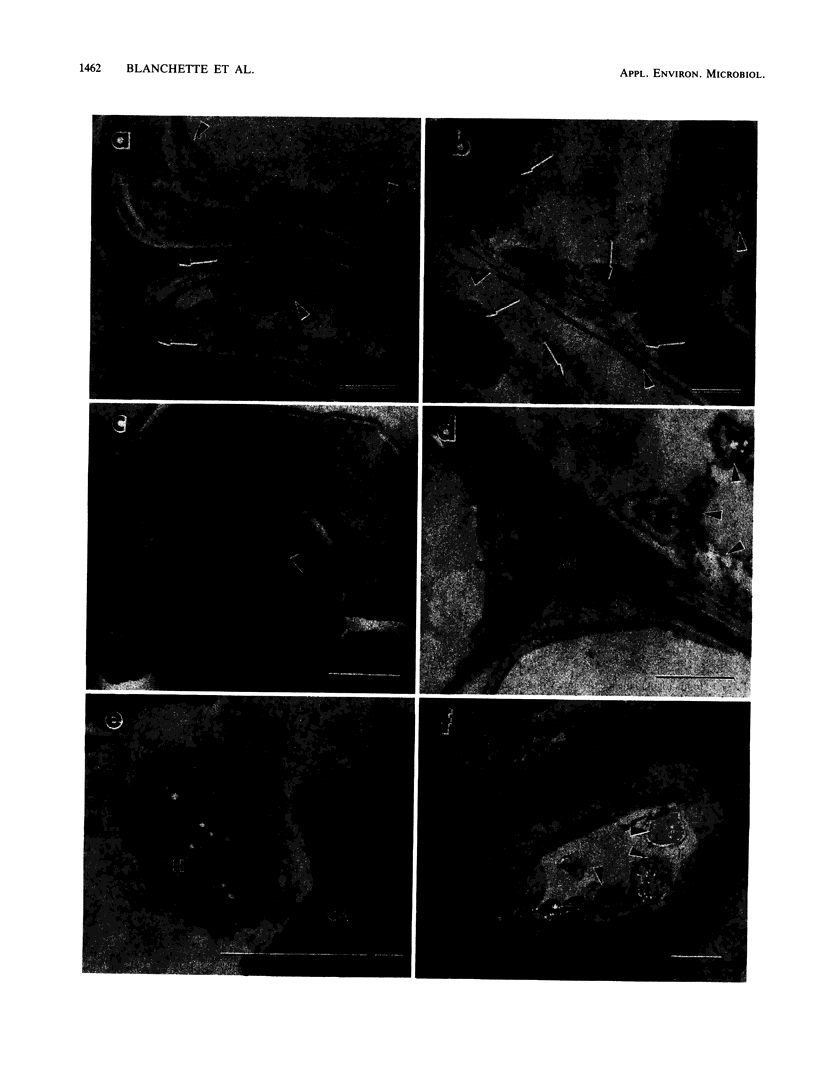
The white rot fungi used in this study caused two different forms of degradation. Phanerochaete chrysosporium, strain BKM-F-1767, and Phellinus pini caused a preferential removal of lignin from birch wood, whereas Trametes (Coriolus) versicolor caused a nonselective attack of all cell wall components. Use of polyclonal antisera to H8 lignin peroxidase and monoclonal antisera to H2 lignin peroxidase followed by immunogold labeling with protein A-gold or protein G-gold, respectively, showed lignin peroxidase extra-and intracellularly to fungal hyphae and within the delignified cell walls after 12 weeks of laboratory decay. Lignin peroxidase was localized at sites within the cell wall where electron-dense areas of the lignified cell wall layers remained. In wood decayed by Trametes versicolor, lignin peroxidase was located primarily along the surface of eroded cell walls. No lignin peroxidase was evident in brown-rotted wood, but slight labeling occurred within hyphal cells. Use of polyclonal antisera to xylanase followed by immunogold labeling showed intense labeling on fungal hyphae and surrounding slime layers and within the woody cell wall, where evidence of degradation was apparent. Colloidal-gold-labeled xylanase was prevalent in wood decayed by all fungi used in this study. Areas of the wood with early stages of cell wall decay had the greatest concentration of gold particles, while little labeling occurred in cells in advanced stages of decay by brown or white rot fungi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asther M., Capdevila C., Corrieu G. Control of Lignin Peroxidase Production by Phanerochaete chrysosporium INA-12 by Temperature Shifting. Appl Environ Microbiol. 1988 Dec;54(12):3194–3196. doi: 10.1128/aem.54.12.3194-3196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M., Nanci A., Kan F. W. Effect of tissue processing on colloidal gold cytochemistry. J Histochem Cytochem. 1987 Sep;35(9):983–996. doi: 10.1177/35.9.3302022. [DOI] [PubMed] [Google Scholar]
- Blanchette R. A., Reid I. D. Ultrastructural Aspects of Wood Delignification by Phlebia (Merulius) tremellosus. Appl Environ Microbiol. 1986 Aug;52(2):239–245. doi: 10.1128/aem.52.2.239-245.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette R. A. Screening wood decayed by white rot fungi for preferential lignin degradation. Appl Environ Microbiol. 1984 Sep;48(3):647–653. doi: 10.1128/aem.48.3.647-653.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G., Nilsson T., Pettersson B. Intra- and Extracellular Localization of Lignin Peroxidase during the Degradation of Solid Wood and Wood Fragments by Phanerochaete chrysosporium by Using Transmission Electron Microscopy and Immuno-Gold Labeling. Appl Environ Microbiol. 1989 Apr;55(4):871–881. doi: 10.1128/aem.55.4.871-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill I., Kraepelin G. Palo Podrido: Model for Extensive Delignification of Wood by Ganoderma applanatum. Appl Environ Microbiol. 1986 Dec;52(6):1305–1312. doi: 10.1128/aem.52.6.1305-1312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Garcia S., Latge J. P., Prevost M. C., Leisola M. Wood degradation by white rot fungi: cytochemical studies using lignin peroxidase-immunoglobulin-gold complexes. Appl Environ Microbiol. 1987 Oct;53(10):2384–2387. doi: 10.1128/aem.53.10.2384-2387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kushida H. Letter: A new method for embedding with a low viscosity epoxy resin "Quetol 651". J Electron Microsc (Tokyo) 1974;23(3):197–197. [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Ruel K., Joseleau J. P. Use of enzyme-gold complexes for the ultrastructural localization of hemicelluloses in the plant cell wall. Histochemistry. 1984;81(6):573–580. doi: 10.1007/BF00489537. [DOI] [PubMed] [Google Scholar]
- Shaw NM, Blanis D, Bodek A, Budd H, Coombes R, Eno S, Fry CA, Harada H, Ho YH, Kim YK. Search for unstable heavy neutral leptons in e+e- annihilations at sqrt s from 50 to 60.8 GeV. Phys Rev Lett. 1989 Sep 25;63(13):1342–1345. doi: 10.1103/PhysRevLett.63.1342. [DOI] [PubMed] [Google Scholar]
- Srebotnik E., Messner K., Foisner R. Penetrability of White Rot-Degraded Pine Wood by the Lignin Peroxidase of Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Nov;54(11):2608–2614. doi: 10.1128/aem.54.11.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]