Abstract
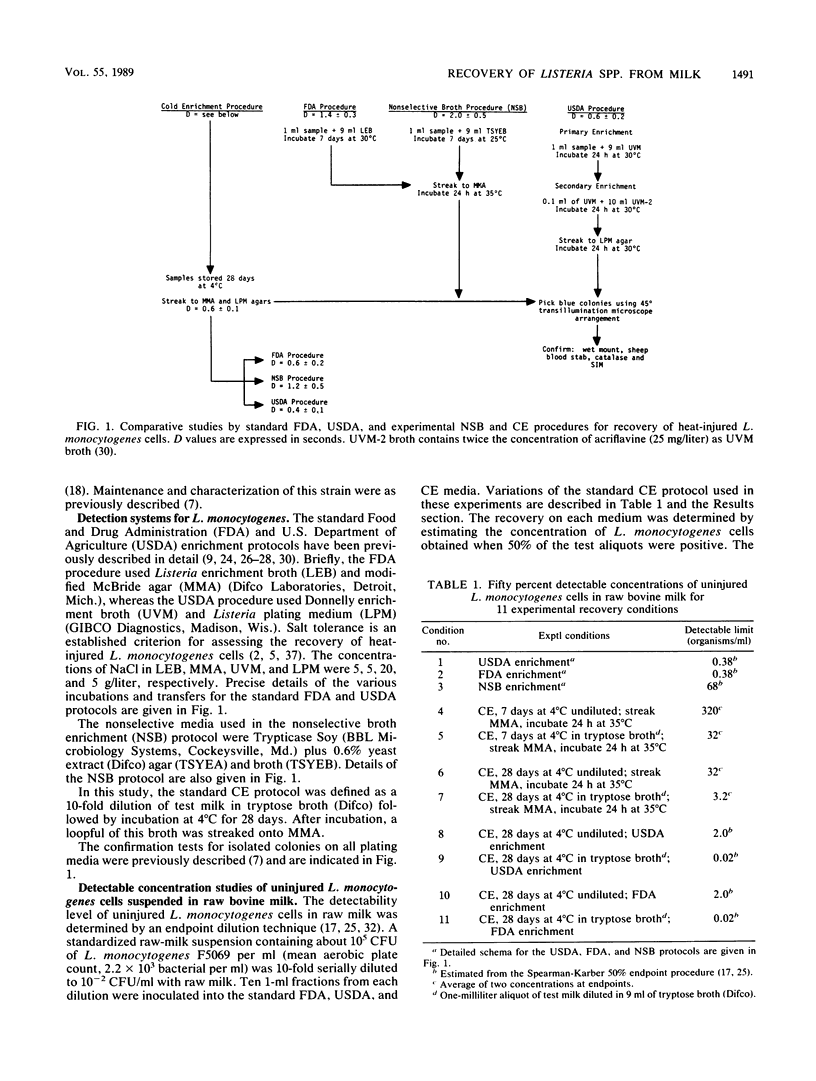
The standard selective enrichment protocols of the Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) were compared with an experimental nonselective broth enrichment (NSB) protocol and variations of the standard cold-enrichment (CE) protocol for the recovery of heat-injured Listeria monocytogenes. Bacterial cells (10(7)/ml) were suspended in sterile milk and heated at 71.7 degrees C in a slug-flow heat exchanger for holding times ranging from 1 to 30 s. Surviving cells were determined (50% endpoint) by the given protocols, and the following D values were obtained: NSB, D = 2.0 +/- 0.5 s; FDA, D = 1.4 +/- 0.3 s; USDA, D = 0.6 +/- 0.2 s; CE, D less than or equal to 1.2 s. The respective direct-plating media used in these enrichments were also analyzed for recovery, and the following D values were calculated from the enumeration of surviving cells; NSB, D = 2.7 +/- 0.8 s; FDA, D = 1.3 +/- 0.4 s; USDA, D = 0.7 +/- 0.2 s. The low levels of heat-injured L. monocytogenes cells which were detected at inactivation endpoints on the optimal nonselective media (25 degrees C for 7 days) failed to recover and multiply during experimental CEs (4 degrees C for 28 days). Initial inactivation experiments in which raw whole milk was used as the heating menstruum gave much lower recoveries with all protocols. The detectable limits for uninjured cells that were suspended in raw milk were similar (0.35 to 3.2 cells per ml) for the standard CE, FDA, and USDA protocols.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannerman E. S., Bille J. A new selective medium for isolating Listeria spp. from heavily contaminated material. Appl Environ Microbiol. 1988 Jan;54(1):165–167. doi: 10.1128/aem.54.1.165-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchat L. R., Brackett R. E., Hao D. Y., Conner D. E. Growth and thermal inactivation of Listeria monocytogenes in cabbage and cabbage juice. Can J Microbiol. 1986 Oct;32(10):791–795. doi: 10.1139/m86-145. [DOI] [PubMed] [Google Scholar]
- Buchanan R. L., Smith J. L., Stahl H. G., Archer D. L. Listeria methods development research at the Eastern Regional Research Center, U.S. Department of Agriculture. J Assoc Off Anal Chem. 1988 May-Jun;71(3):651–654. [PubMed] [Google Scholar]
- Bunning V. K., Crawford R. G., Bradshaw J. G., Peeler J. T., Tierney J. T., Twedt R. M. Thermal resistance of intracellular Listeria monocytogenes cells suspended in raw bovine milk. Appl Environ Microbiol. 1986 Dec;52(6):1398–1402. doi: 10.1128/aem.52.6.1398-1402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning V. K., Donnelly C. W., Peeler J. T., Briggs E. H., Bradshaw J. G., Crawford R. G., Beliveau C. M., Tierney J. T. Thermal inactivation of Listeria monocytogenes within bovine milk phagocytes. Appl Environ Microbiol. 1988 Feb;54(2):364–370. doi: 10.1128/aem.54.2.364-370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez Rodriguez L., Fernández Garayzabal J. F., Vazquez Boland J. A., Rodriguez Ferri E., Suarez Fernández G. Isolation de micro-organismes du genre Listeria à partir de lait cru destiné à la consommation humaine. Can J Microbiol. 1985 Oct;31(10):938–941. [PubMed] [Google Scholar]
- Donnelly C. W., Baigent G. J. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl Environ Microbiol. 1986 Oct;52(4):689–695. doi: 10.1128/aem.52.4.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. W. Historical perspectives on methodology to detect Listeria monocytogenes. J Assoc Off Anal Chem. 1988 May-Jun;71(3):644–646. [PubMed] [Google Scholar]
- Doyle M. P., Glass K. A., Beery J. T., Garcia G. A., Pollard D. J., Schultz R. D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl Environ Microbiol. 1987 Jul;53(7):1433–1438. doi: 10.1128/aem.53.7.1433-1438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Sanders G. W., Malcolm S. A. The presence of Listeria spp. in raw milk in Ontario. Can J Microbiol. 1988 Feb;34(2):95–100. doi: 10.1139/m88-020. [DOI] [PubMed] [Google Scholar]
- Fernandez Garayzabal J. F., Dominguez Rodriguez L., Vazquez Boland J. A., Rodriguez Ferri E. F., Briones Dieste V., Blanco Cancelo J. L., Suarez Fernandez G. Survival of Listeria monocytogenes in raw milk treated in a pilot plant size pasteurizer. J Appl Bacteriol. 1987 Dec;63(6):533–537. doi: 10.1111/j.1365-2672.1987.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Fricker C. R. The isolation of salmonellas and campylobacters. J Appl Bacteriol. 1987 Aug;63(2):99–116. doi: 10.1111/j.1365-2672.1987.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Golden D. A., Beuchat L. R., Brackett R. E. Evaluation of selective direct plating media for their suitability to recover uninjured, heat-injured, and freeze-injured Listeria monocytogenes from foods. Appl Environ Microbiol. 1988 Jun;54(6):1451–1456. doi: 10.1128/aem.54.6.1451-1456.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P. S., Feeley J. C., Graves L. M., Ajello G. W., Fleming D. W. Isolation of Listeria monocytogenes from raw milk. Appl Environ Microbiol. 1986 Feb;51(2):438–440. doi: 10.1128/aem.51.2.438-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS J. C. The estimation of decimal reduction times. Appl Microbiol. 1956 Jul;4(4):211–221. doi: 10.1128/am.4.4.211-221.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., McClain D. Improved Listeria monocytogenes selective agar. Appl Environ Microbiol. 1986 Nov;52(5):1215–1217. doi: 10.1128/aem.52.5.1215-1217.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. Isolation and identification of Listeria monocytogenes in dairy products. J Assoc Off Anal Chem. 1988 May-Jun;71(3):658–660. [PubMed] [Google Scholar]
- McClain D., Lee W. H. Development of USDA-FSIS method for isolation of Listeria monocytogenes from raw meat and poultry. J Assoc Off Anal Chem. 1988 May-Jun;71(3):660–664. [PubMed] [Google Scholar]
- Ralovich B., Forray A., Mérö E., Málovics H., Százados I. New selective medium for isolation of L. monocytogenes. Zentralbl Bakteriol Orig. 1971;216(1):88–91. [PubMed] [Google Scholar]
- Shahamat M., Seaman A., Woodbine M. Survival of Listeria monocytogenes in high salt concentrations. Zentralbl Bakteriol A. 1980;246(4):506–511. [PubMed] [Google Scholar]
- Stroup W. H., Dickerson R. W., Jr, Read R. B., Jr Two-phase slug flow heat exchanger for microbial thermal inactivation research. Appl Microbiol. 1969 Nov;18(5):889–892. doi: 10.1128/am.18.5.889-892.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


