Abstract
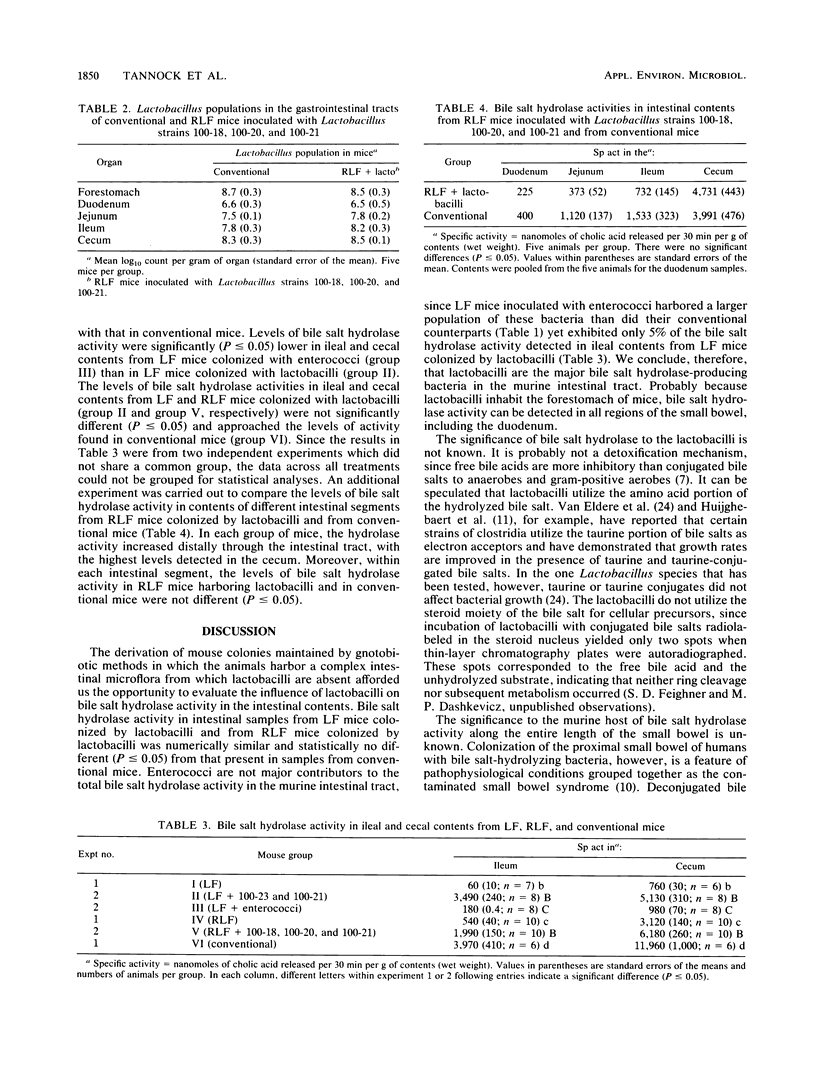
Mice that have a complex intestinal microflora but that do not harbor lactobacilli were used to determine the contribution of lactobacilli to the total bile salt hydrolase activity in the murine intestinal tract. Bile salt hydrolase activity in the ileal contents of these mice was reduced 86% in the absence of lactobacilli and by greater than 98% in the absence of lactobacilli and enterococci compared with samples from conventional mice. Bile salt hydrolase activities were lower in ileal and cecal contents from lactobacillus-free mice colonized with enterococci than in samples from lactobacillus-free mice colonized with lactobacilli. Bile salt hydrolase activity in the duodena, jejuna, ilea, and ceca of reconstituted lactobacillus-free mice colonized by lactobacilli was similar to that in samples from the intestinal tracts of conventional mice. We conclude from these studies that lactobacilli are the main contributors to total bile salt hydrolase activity in the murine intestinal tract.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. B., Fuller R. Bile acid deconjugation and attachment of chicken gut bacteria: their possible role in growth depression. Br Poult Sci. 1984 Apr;25(2):227–231. doi: 10.1080/00071668408454861. [DOI] [PubMed] [Google Scholar]
- Dashkevicz M. P., Feighner S. D. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol. 1989 Jan;55(1):11–16. doi: 10.1128/aem.55.1.11-16.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Effect of dietary carbohydrates on bacterial cholyltaurine hydrolase in poultry intestinal homogenates. Appl Environ Microbiol. 1988 Feb;54(2):337–342. doi: 10.1128/aem.54.2.337-342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl Environ Microbiol. 1987 Feb;53(2):331–336. doi: 10.1128/aem.53.2.331-336.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floch M. H., Binder H. J., Filburn B., Gershengoren W. The effect of bile acids on intestinal microflora. Am J Clin Nutr. 1972 Dec;25(12):1418–1426. doi: 10.1093/ajcn/25.12.1418. [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Deconjugation of bile acids by intestinal lactobacilli. Appl Environ Microbiol. 1977 Jan;33(1):15–18. doi: 10.1128/aem.33.1.15-18.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijghebaert S. M., Mertens J. A., Eyssen H. J. Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microflora. Appl Environ Microbiol. 1982 Jan;43(1):185–192. doi: 10.1128/aem.43.1.185-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach S., Savage D. C., Tannock G. W. Lactobacilli isolated from the stomach of conventional mice. Appl Environ Microbiol. 1977 May;33(5):1197–1203. doi: 10.1128/aem.33.5.1197-1203.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach S., Tannock G. W. Indigenous bacteria influence the number of Salmonella typhimurium in the ileum of gnotobiotic mice. Can J Microbiol. 1979 Dec;25(12):1352–1358. doi: 10.1139/m79-213. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Archibald R. D. The derivation and use of mice which do not harbour lactobacilli in the gastrointestinal tract. Can J Microbiol. 1984 Jun;30(6):849–853. doi: 10.1139/m84-131. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Crichton C., Welling G. W., Koopman J. P., Midtvedt T. Reconstitution of the gastrointestinal microflora of lactobacillus-free mice. Appl Environ Microbiol. 1988 Dec;54(12):2971–2975. doi: 10.1128/aem.54.12.2971-2975.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Szylit O., Duval Y., Raibaud P. Colonization of tissue surfaces in the gastrointestinal tract of gnotobiotic animals by lactobacillus strains. Can J Microbiol. 1982 Oct;28(10):1196–1198. doi: 10.1139/m82-177. [DOI] [PubMed] [Google Scholar]
- Van Eldere J., Robben J., De Pauw G., Merckx R., Eyssen H. Isolation and identification of intestinal steroid-desulfating bacteria from rats and humans. Appl Environ Microbiol. 1988 Aug;54(8):2112–2117. doi: 10.1128/aem.54.8.2112-2117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


