Abstract
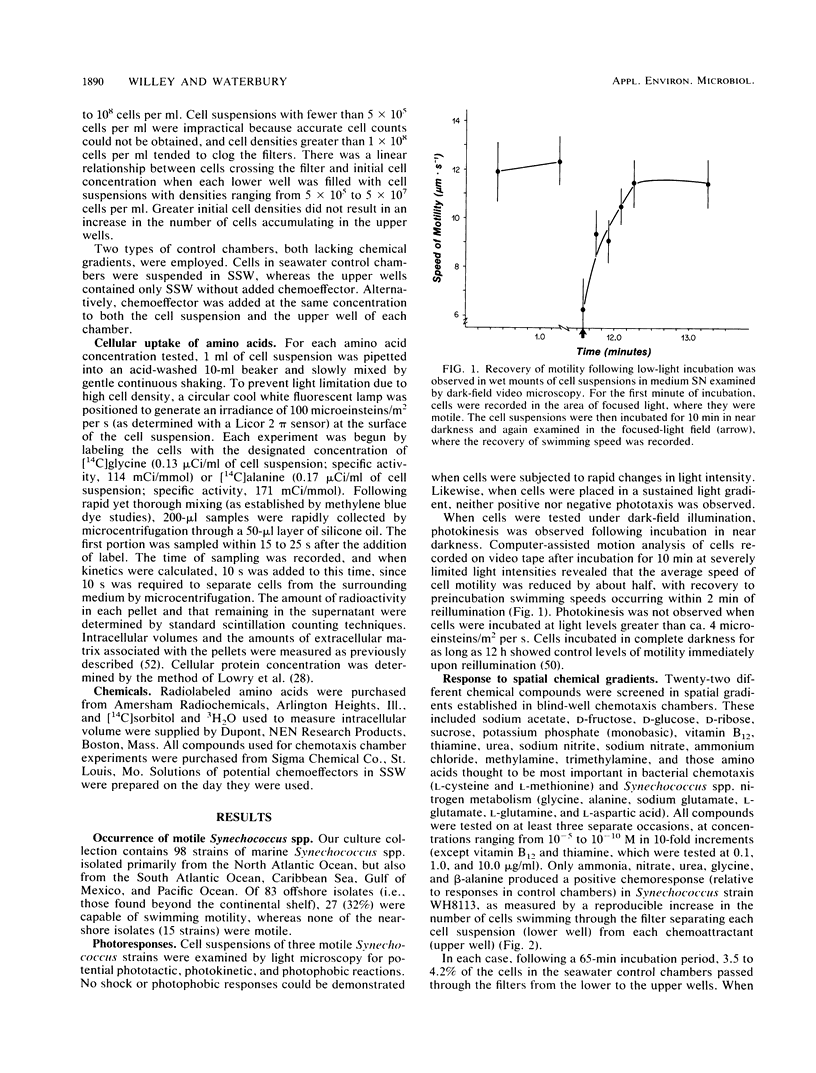
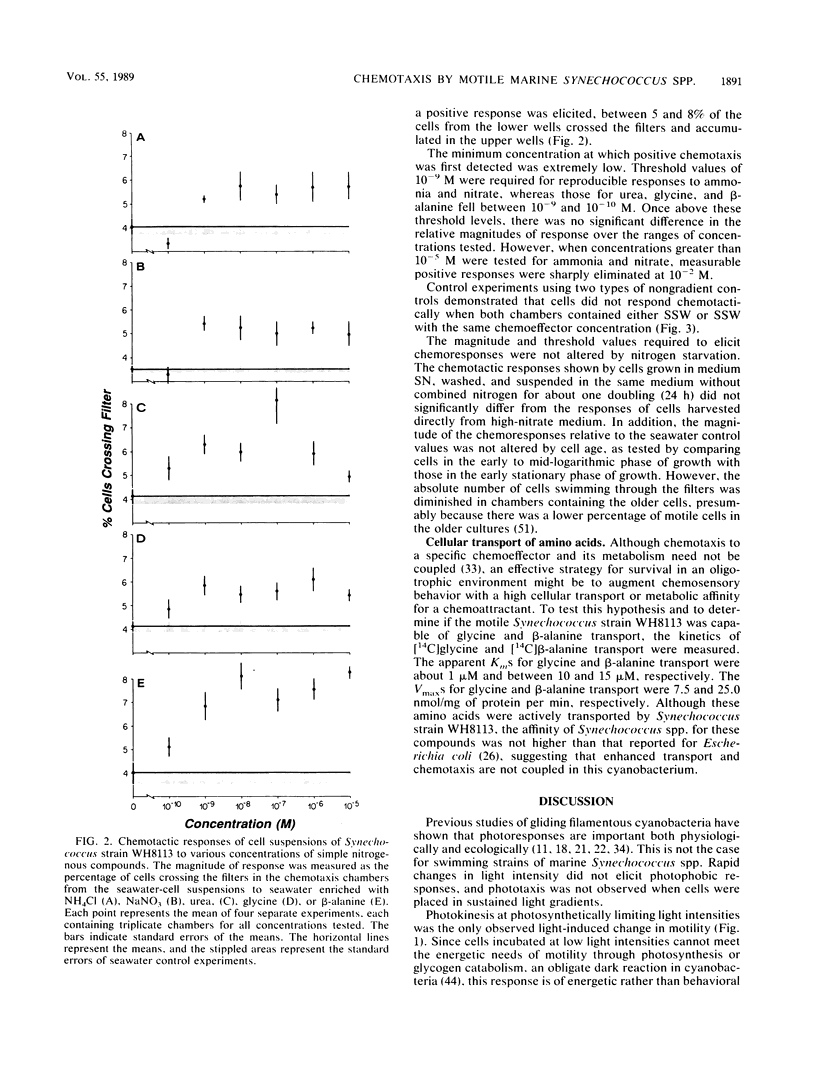
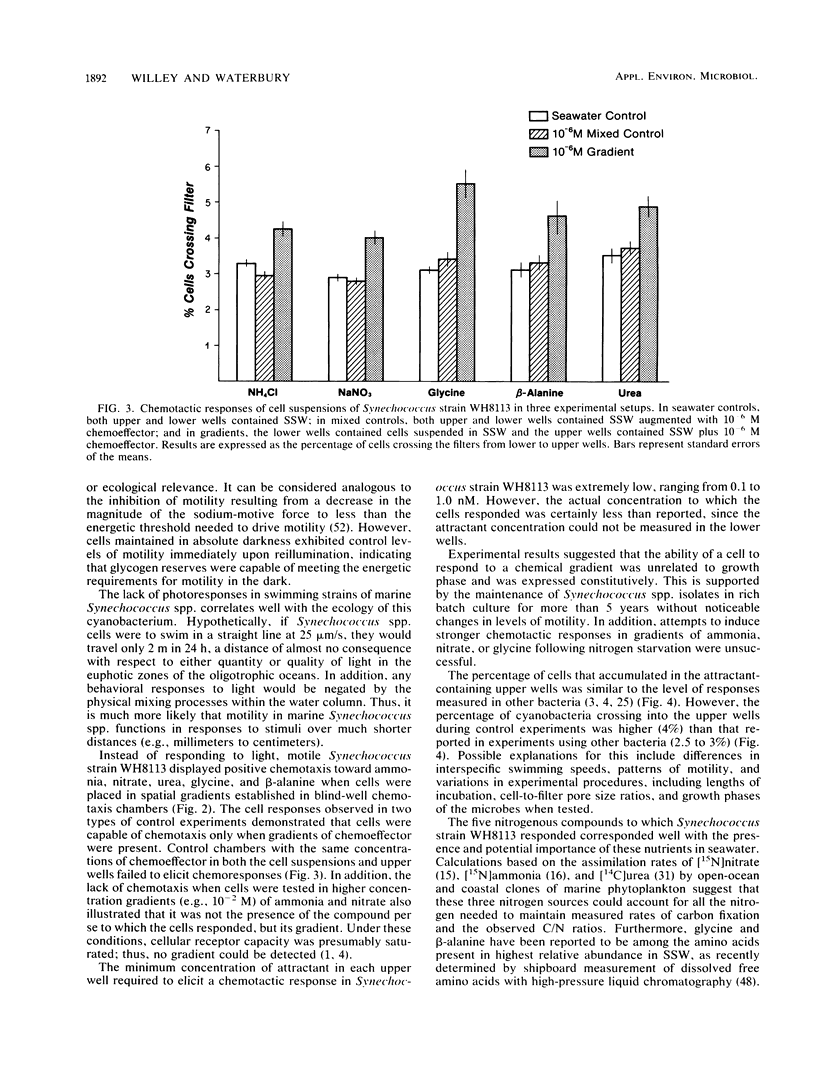
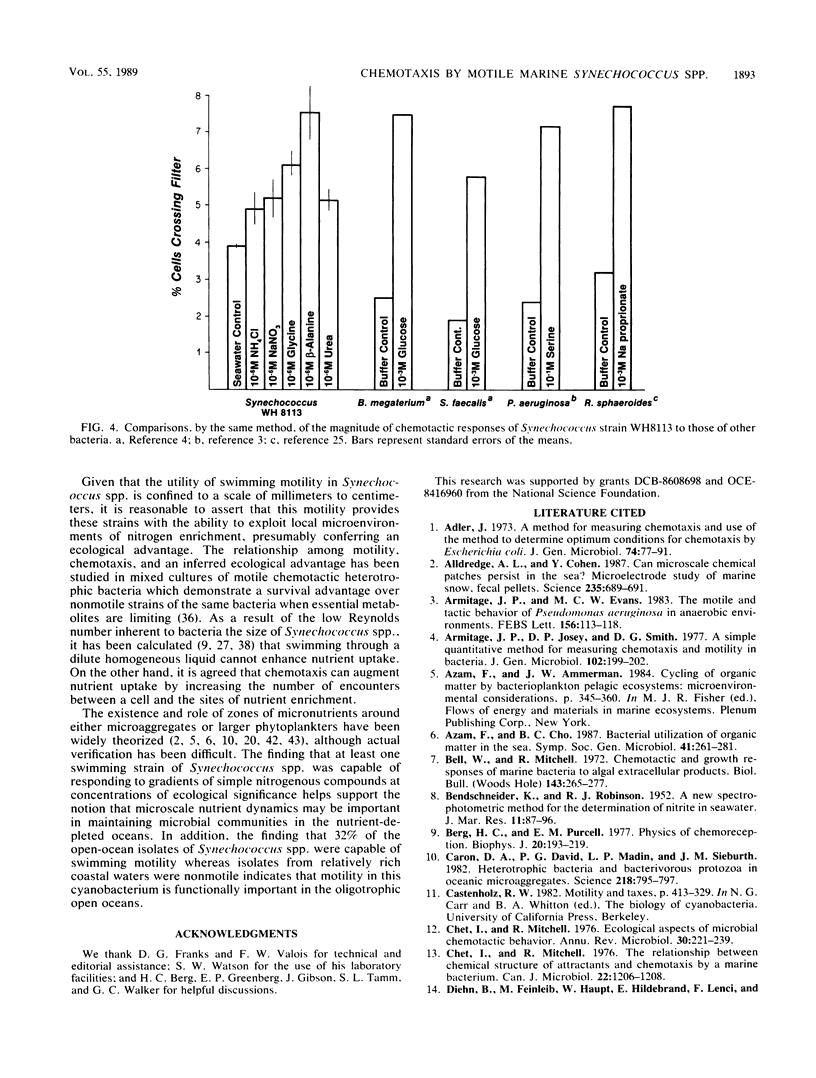
Many of the open-ocean isolates of the marine unicellular cyanobacterium Synechococcus spp. are capable of swimming motility, whereas coastal isolates are nonmotile. Surprisingly, the motile strains do not display phototactic or photophobic responses to light, but they do demonstrate positive chemoresponses to several nitrogenous compounds. The chemotactic responses of Synechococcus strain WH8113 were investigated using blind-well chemotaxis chambers fitted with 3.0-μm-pore-size Nuclepore filters. One well of each chamber contained cells suspended in aged Sargasso Sea water, and the other well contained the potential chemoattractant in seawater. The number of cells that crossed the filter into the attractant-seawater mixture was measured by direct cell counts and compared with values obtained in chambers lacking gradients. Twenty-two compounds were tested, including sugars, amino acids, and simple nitrogenous substrates, at concentrations ranging from 10−5 to 10−10 M. Strain WH8113 responded positively only to ammonia, nitrate, β-alanine, glycine, and urea. Typically, there was a 1.5- to 2-fold increase in cell concentrations above control levels in chambers containing these compounds, which is comparable to results from similar experiments using enteric and photoheterotrophic bacteria. However, the threshold levels of 10−9 to 10−10 M found for Synechococcus spp. chemoresponses were lower by several orders of magnitude than those reported for other bacteria and fell within a range that could be ecologically significant in the oligotrophic oceans. The presence of chemotaxis in motile Synechococcus spp. supports the notion that regions of nutrient enrichment, such as the proposed microzones and patches, may play an important role in picoplankton nutrient dynamics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Alldredge A. L., Cohen Y. Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science. 1987 Feb 6;235(4789):689–691. doi: 10.1126/science.235.4789.689. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. The motile and tactic behaviour of Pseudomonas aeruginosa in anaerobic environments. FEBS Lett. 1983 May 30;156(1):113–118. doi: 10.1016/0014-5793(83)80259-2. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A., Davis P. G., Madin L. P., Sieburth J. M. Heterotrophic bacteria and bacterivorous protozoa in oceanic macroaggregates. Science. 1982 Nov 19;218(4574):795–797. doi: 10.1126/science.218.4574.795. [DOI] [PubMed] [Google Scholar]
- Chet I., Mitchell R. Ecological aspects of microbial chemotactic behavior. Annu Rev Microbiol. 1976;30:221–239. doi: 10.1146/annurev.mi.30.100176.001253. [DOI] [PubMed] [Google Scholar]
- Chet I., Mitchell R. The relationship between chemical structure of attractants and chemotaxis by a marine bacterium. Can J Microbiol. 1976 Aug;22(8):1206–1208. doi: 10.1139/m76-178. [DOI] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Häder D. P. Photosensory behavior in procaryotes. Microbiol Rev. 1987 Mar;51(1):1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham C. J., Armitage J. P. Involvement of transport in Rhodobacter sphaeroides chemotaxis. J Bacteriol. 1987 Dec;169(12):5801–5807. doi: 10.1128/jb.169.12.5801-5807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauffenburger D., Aris R., Keller K. Effects of cell motility and chemotaxis on microbial population growth. Biophys J. 1982 Dec;40(3):209–219. doi: 10.1016/S0006-3495(82)84476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. J., Goldman J. C. Nitrogenous nutrition of marine phytoplankton in nutrient-depleted waters. Science. 1979 Feb 16;203(4381):670–672. doi: 10.1126/science.203.4381.670. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgram W. K., Williams F. D. Survival value of chemotaxis in mixed cultures. Can J Microbiol. 1976 Dec;22(12):1771–1773. doi: 10.1139/m76-262. [DOI] [PubMed] [Google Scholar]
- Richardson L. L., Castenholz R. W. Chemokinetic Motility Responses of the Cyanobacterium Oscillatoria terebriformis. Appl Environ Microbiol. 1989 Jan;55(1):261–263. doi: 10.1128/aem.55.1.261-263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L. L., Castenholz R. W. Diel Vertical Movements of the Cyanobacterium Oscillatoria terebriformis in a Sulfide-Rich Hot Spring Microbial Mat. Appl Environ Microbiol. 1987 Sep;53(9):2142–2150. doi: 10.1128/aem.53.9.2142-2150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. W., Shanks A. L., Trent J. D. Marine snow: microplankton habitat and source of small-scale patchiness in pelagic populations. Science. 1978 Jul 28;201(4353):371–373. doi: 10.1126/science.201.4353.371. [DOI] [PubMed] [Google Scholar]
- Sundberg S. A., Bogomolni R. A., Spudich J. L. Selection and properties of phototaxis-deficient mutants of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):282–287. doi: 10.1128/jb.164.1.282-287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterbury J. B., Willey J. M., Franks D. G., Valois F. W., Watson S. W. A cyanobacterium capable of swimming motility. Science. 1985 Oct 4;230(4721):74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]
- Willey J. M., Waterbury J. B., Greenberg E. P. Sodium-coupled motility in a swimming cyanobacterium. J Bacteriol. 1987 Aug;169(8):3429–3434. doi: 10.1128/jb.169.8.3429-3434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


