Abstract
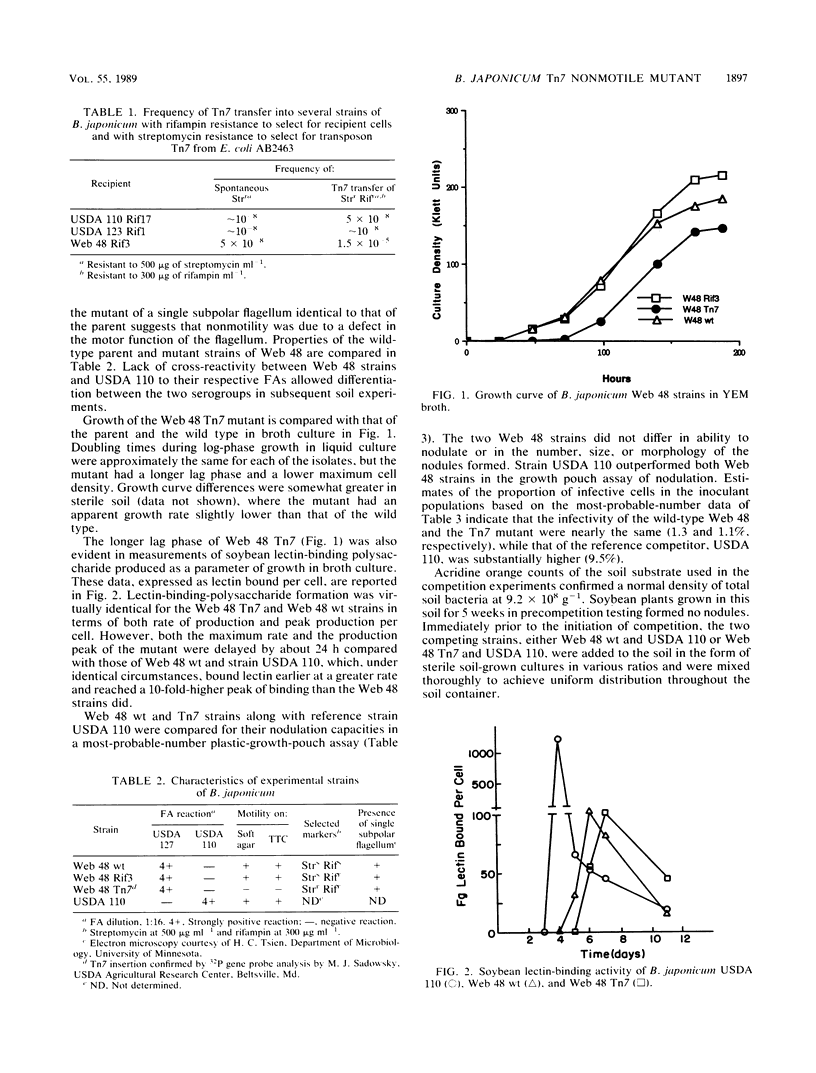
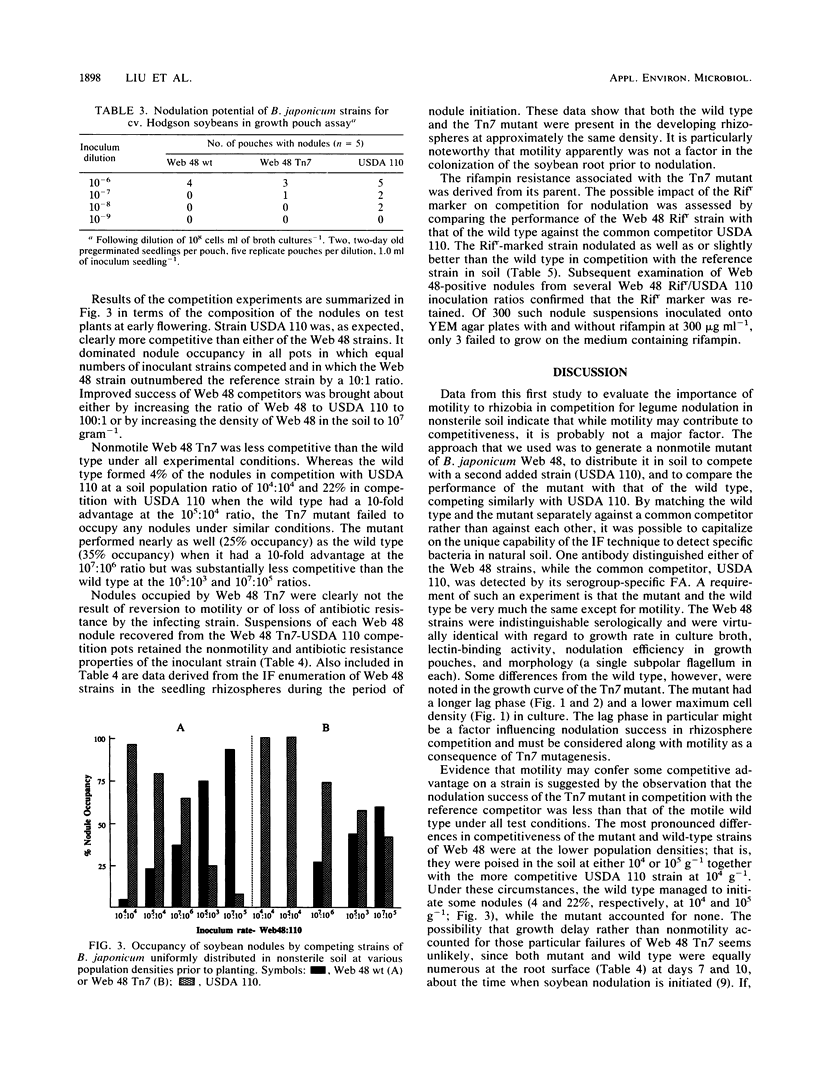
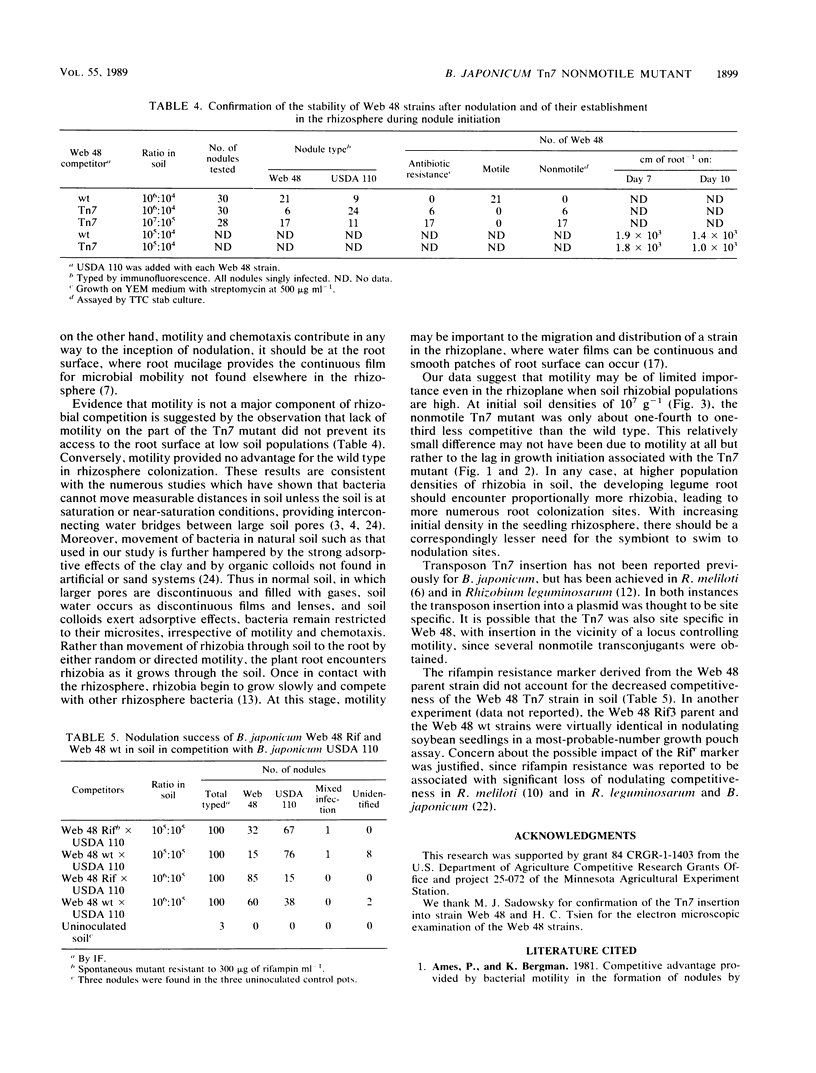
A nonmotile mutant of Bradyrhizobium japonicum serogroup 127 was generated by Tn7 mutagenesis and matched with the wild type against a common competitor in studies of soybean nodulation in nonsterile soil. The Tn7 mutant was very similar to the wild type in growth rate in culture, soybean lectin-binding ability, flagellar morphology, and nodulating capability, but it had a longer lag phase. Competing strains were distributed uniformly in soil in various ratios and at different population densities prior to planting. Mutant and wild type were equally prevalent in the seedling rhizosphere at about the time of nodule initiation, suggesting that motility conferred no advantage in rhizosphere colonization. Nodulation success of the Tn7 mutant was lower than that of the wild type under all test conditions. Differences were greatest at low soil populations of competitors and much less pronounced at initial populations of 107 g−1. The longer lag phase of the Tn7 mutant may have contributed to its decreased competitiveness, especially at the higher inoculation levels. The antibiotic and motility markers were stable, and the rifampin resistance derived from the parent did not affect adversely the competitiveness of the Tn7 mutant. We found motility to be of limited importance to the competitiveness of a strain in normal nonsterile soil, where the significance, if any, of this ability may be in migration at the immediate root surface in soils sparsely populated with rhizobial symbionts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., Schluederberg S. A., Bergman K. Behavioral mutants of Rhizobium meliloti. J Bacteriol. 1980 Feb;141(2):722–727. doi: 10.1128/jb.141.2.722-727.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Serological diversity within a terrestrial ammonia-oxidizing population. Appl Environ Microbiol. 1978 Oct;36(4):589–593. doi: 10.1128/aem.36.4.589-593.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B., Dowdle S., Sadowsky M. J. Competition of Rhizobium japonicum Strains in Early Stages of Soybean Nodulation. Appl Environ Microbiol. 1983 Oct;46(4):870–873. doi: 10.1128/aem.46.4.870-873.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Albersheim P. Infection and nodulation of clover by nonmotile Rhizobium trifolii. J Bacteriol. 1980 Feb;141(2):979–980. doi: 10.1128/jb.141.2.979-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soby S., Bergman K. Motility and Chemotaxis of Rhizobium meliloti in Soil. Appl Environ Microbiol. 1983 Nov;46(5):995–998. doi: 10.1128/aem.46.5.995-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Accumulation of Soybean Lectin-Binding Polysaccharide During Growth of Rhizobium japonicum as Determined by Hemagglutination Inhibition Assay. Appl Environ Microbiol. 1980 Jun;39(6):1100–1104. doi: 10.1128/aem.39.6.1100-1104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


