Abstract
The fluorescent pseudomonads are classified as a group, one characteristic of which is that they do not accumulate poly-3-hydroxybutyrate (PHB) during nutrient starvation in the presence of excess carbon source. In this paper we show that prototype strains from this subclass, such as Pseudomonas aeruginosa, Pseudomonas putida, and Pseudomonas fluorescens, do accumulate poly-3-hydroxyalkanoates (PHA) when grown on fatty acids. These PHAs are composed of medium-chain-length (C6 to C12) 3-hydroxy fatty acids. The ability to form these polyesters does not depend on the presence of plasmids. A specificity profile of the enzymes involved in the biosynthesis of PHA was determined by growing Pseudomonas oleovorans on fatty acids ranging from C4 to C18. In all cases, PHAs were formed which contained C6 to C12 3-hydroxy fatty acids, with a strong preference for 3-hydroxyoctanoate when Ceven fatty acids were supplied and 3-hydroxynonanoate when Codd fatty acids were the substrate. These results indicate that the formation of PHAs depends on a specific enzyme system which is distinct from that responsible for the synthesis of PHB. While the fluorescent pseudomonads are characterized by their inability to make PHB, they appear to share the capacity to produce PHAs. This characteristic may be helpful in classifying pseudomonads. It may also be useful in the optimization of PHA production for biopolymer applications.
Full text
PDF

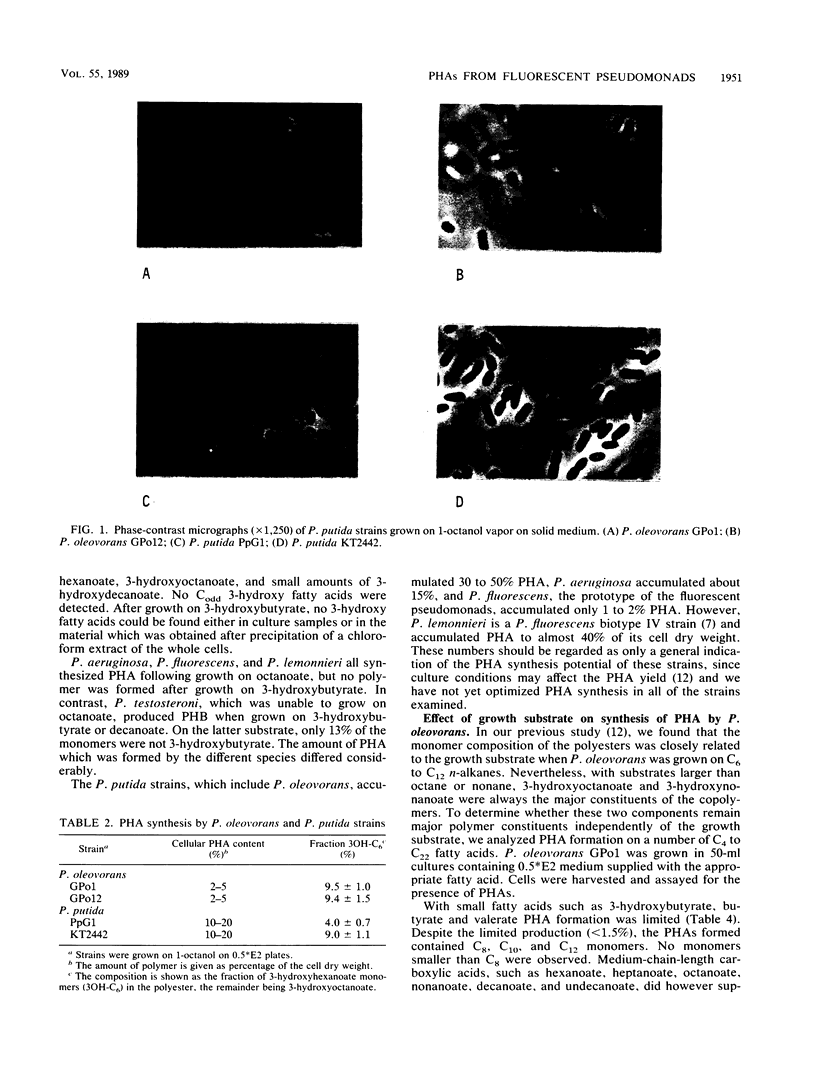
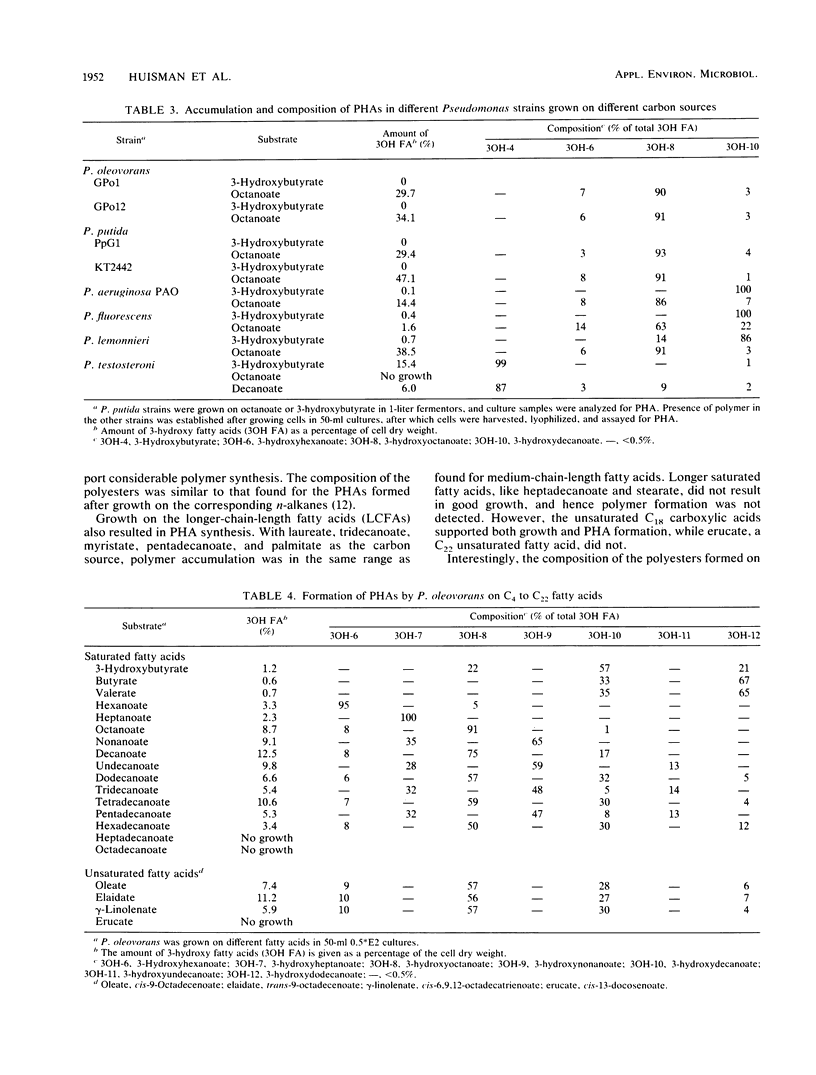
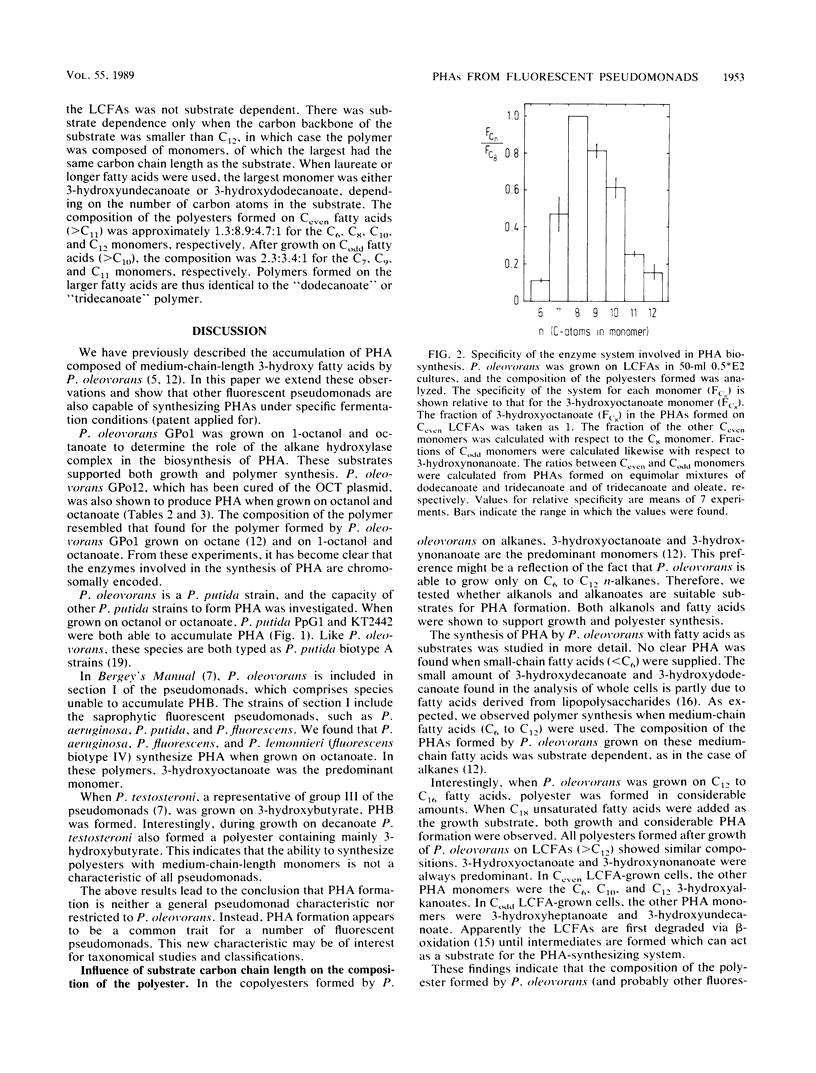

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng G. S., Johnson J. L., Whitaker R. J., Gherna R. L., Jensen R. A. The evolutionary pattern of aromatic amino acid biosynthesis and the emerging phylogeny of pseudomonad bacteria. J Mol Evol. 1983;19(3-4):272–282. doi: 10.1007/BF02099974. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Eggink G., van Lelyveld P. H., Arnberg A., Arfman N., Witteveen C., Witholt B. Structure of the Pseudomonas putida alkBAC operon. Identification of transcription and translation products. J Biol Chem. 1987 May 5;262(13):6400–6406. [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L. S., Nunn W. D. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J Bacteriol. 1987 Jan;169(1):42–52. doi: 10.1128/jb.169.1.42-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS P. I., TALALAY P. Induction and purification of alpha- and beta-hydroxysteroid dehydrogenases. J Biol Chem. 1956 Feb;218(2):661–674. [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Pseudomonas oleovorans hydroxylation-epoxidation system: additional strain improvements. Appl Microbiol. 1973 Aug;26(2):217–218. doi: 10.1128/am.26.2.217-218.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Witholt B. Method for isolating mutants overproducing nicotinamide adenine dinucleotide and its precursors. J Bacteriol. 1972 Jan;109(1):350–364. doi: 10.1128/jb.109.1.350-364.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M. J., Eggink G., Witholt B., Kingma J., Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983 May;154(2):870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]