Abstract
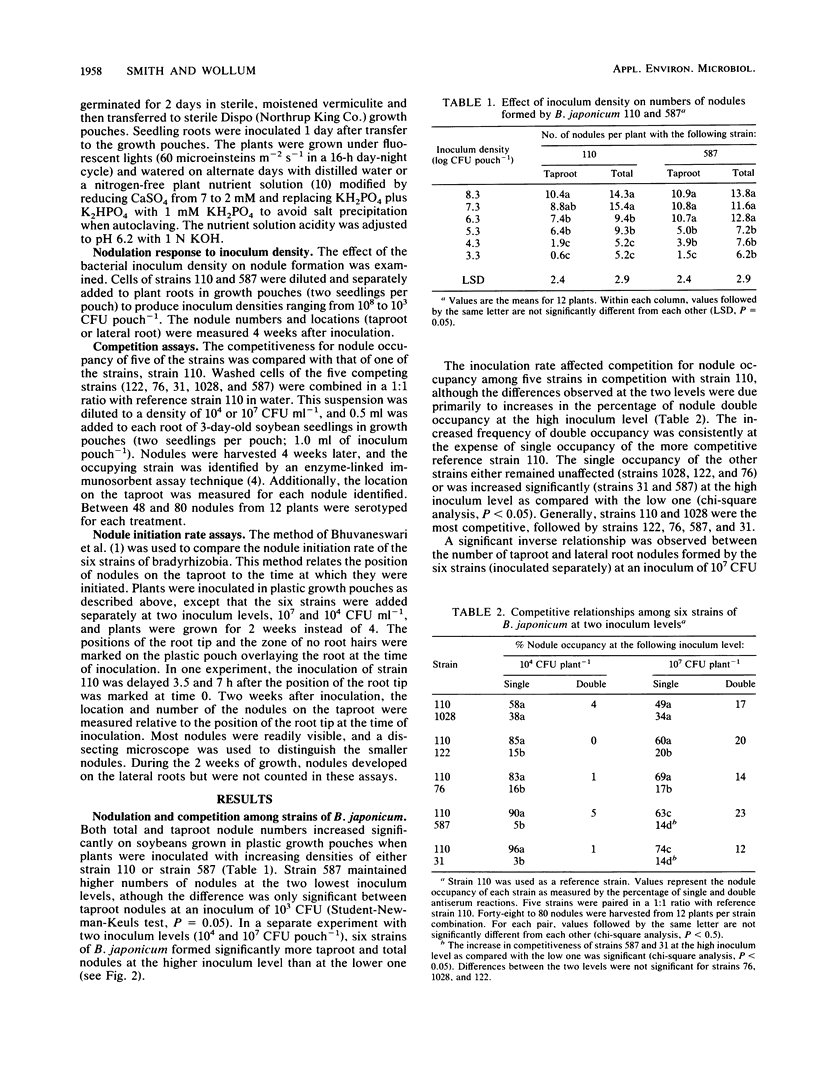
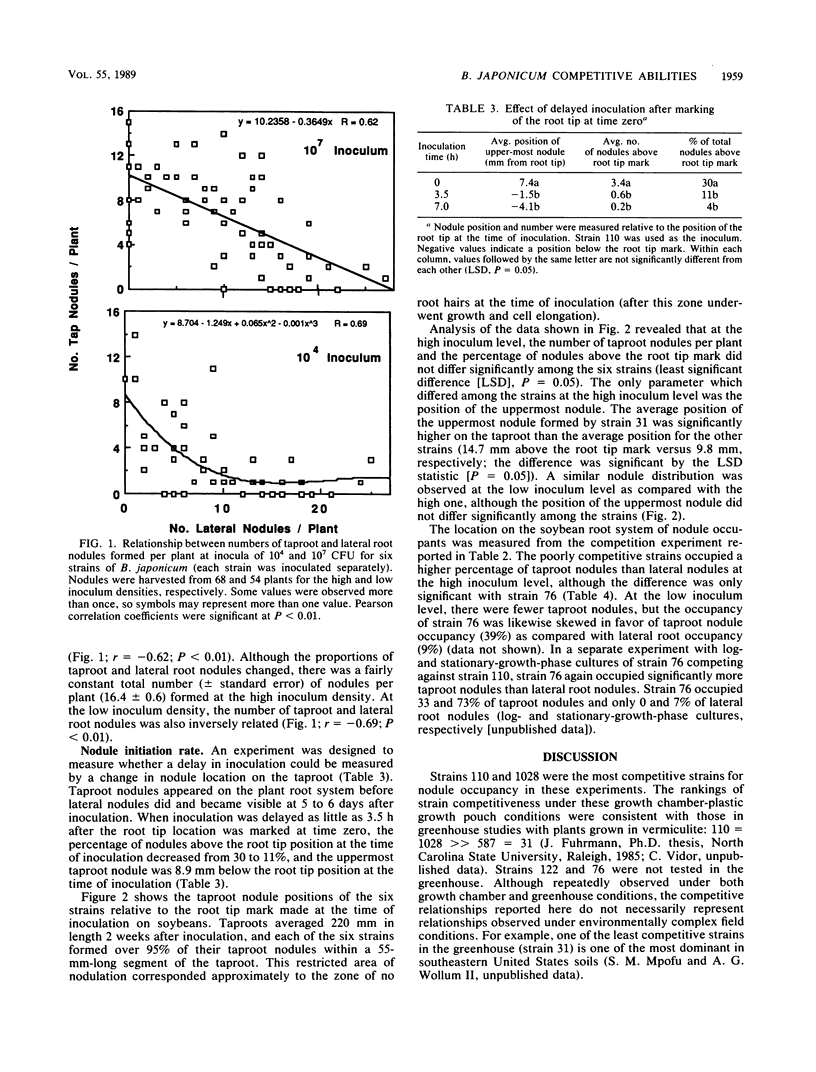
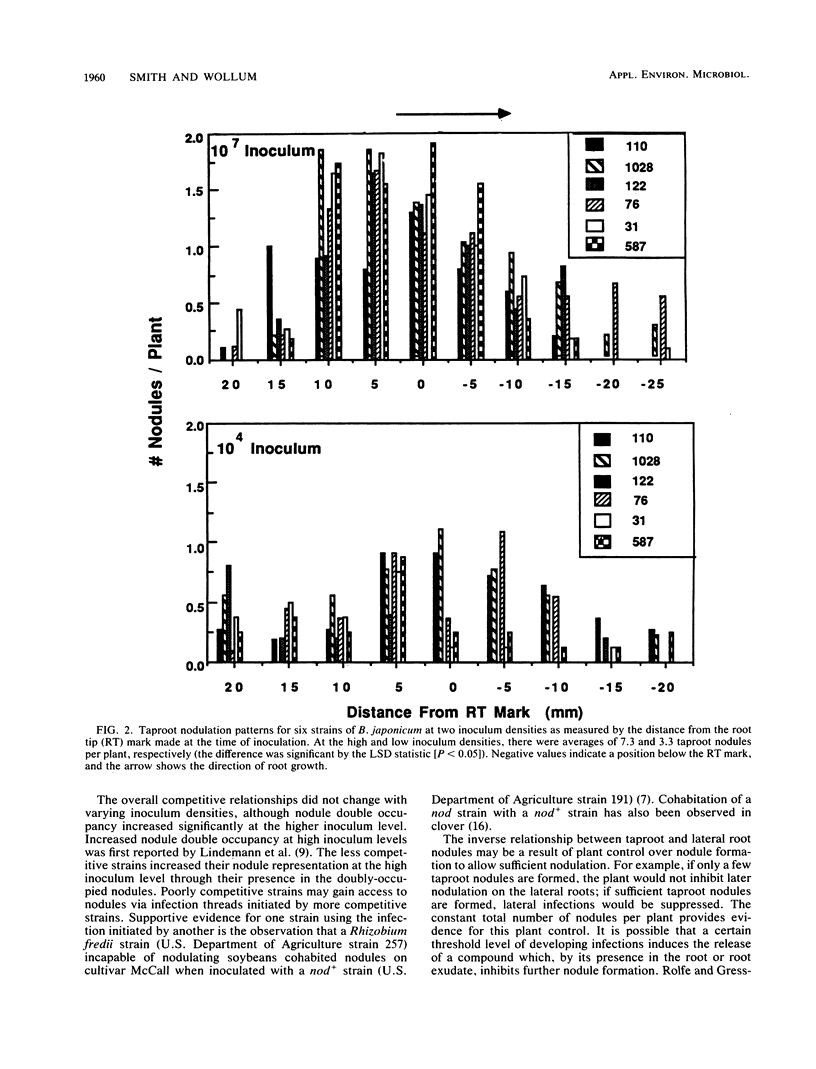
The root nodule locations of six Bradyrhizobium japonicum strains were examined to determine if there were any differences which might explain their varying competitiveness for nodule occupancy on Glycine max. When five strains were added to soybeans in plastic growth pouches in equal proportions with a reference strain (U.S. Department of Agriculture, strain 110), North Carolina strain 1028 and strain 110 were the most competitive for nodule occupancy, followed by U.S. Department of Agriculture strains 122, 76, and 31 and Brazil strain 587. Among all strains, nodule double occupancy was 17% at a high inoculum level (107 CFU pouch−1) and 2% at a low inoculum level (104 CFU pouch−1). The less competitive strains increased their nodule representation by an increase in the doubly occupied nodules at the high inoculum level. Among all strains, the number of taproot and lateral root nodules was inversely related at both the high and low inoculum levels (r = −0.62 and −0.69, respectively; P = 0.0001). This inverse relationship appeared to be a result of the plant host control of bacterial infection. Among each of the six strains, greater than 95% of the taproot nodules formed at the high inoculum density were located on 25% of the taproot length, the nodules centering on the position of the root tip at the time of inoculation. No differences among the six strains were observed in nodule initiation rates as measured by taproot nodule position. Taproot nodules were formed in the symbiosis before lateral root nodules. One of the poorly competitive strains (strain 76) occupied three times as many taproot nodules as lateral root nodules when competing with strain 110 (nodules were harvested from 4-week-old plants). Among these six wild-type strains of B. japonicum, competitive ability evidently is not related to nodule initiation rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A. C., Mathews A., Day D. A., Carter A. S., Carroll B. J., Gresshoff P. M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986 Oct;82(2):588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Matthias, Hennecke Hauke. Cloning and Mapping of a Novel Nodulation Region from Bradyrhizobium japonicum by Genetic Complementation of a Deletion Mutant. Appl Environ Microbiol. 1988 Jan;54(1):55–61. doi: 10.1128/aem.54.1.55-61.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis : evidence for the involvement of lectin in nodulation. Plant Physiol. 1985 Mar;77(3):621–625. doi: 10.1104/pp.77.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron D. S., Pueppke S. G. Regulation of nodulation in the soybean-Rhizobium symbiosis : strain and cultivar variability. Plant Physiol. 1987 Aug;84(4):1391–1396. doi: 10.1104/pp.84.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munévar F., Wollum A. G. Growth of Rhizobium japonicum Strains at Temperatures Above 27 degrees C. Appl Environ Microbiol. 1981 Aug;42(2):272–276. doi: 10.1128/aem.42.2.272-276.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdor R. E., Pueppke S. G. Early Infection and Competition for Nodulation of Soybean by Bradyrhizobium japonicum 123 and 138. Appl Environ Microbiol. 1988 Aug;54(8):1996–2002. doi: 10.1128/aem.54.8.1996-2002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


