Abstract
A fixed-bed loop, high-rate anaerobic bioreactor treating sulfite evaporator condensate was sampled when it reached steady state and afterwards following perturbations during a 14-month period. By using immunotechnology, it was observed that shifts in methanogenic subpopulations occurred in association with perturbations, such as restarting and relocating the biomass into a different tank. Methanogens related to Methanobacterium bryantii MoHG and Methanobrevibacter smithii ALI were numerous throughout the observation period, while Methanosarcina mazei S6 and Methanosarcina thermophila TM1 were found in the early and late samples, respectively. Also, Methanobacterium formicicum was more numerous at the top portion of the bioreactor, while Methanobrevibacter arboriphilus AZ and DC were at the bottom. Sample formalinization required for prolonged storage proved suitable for antigen preservation.
Full text
PDF


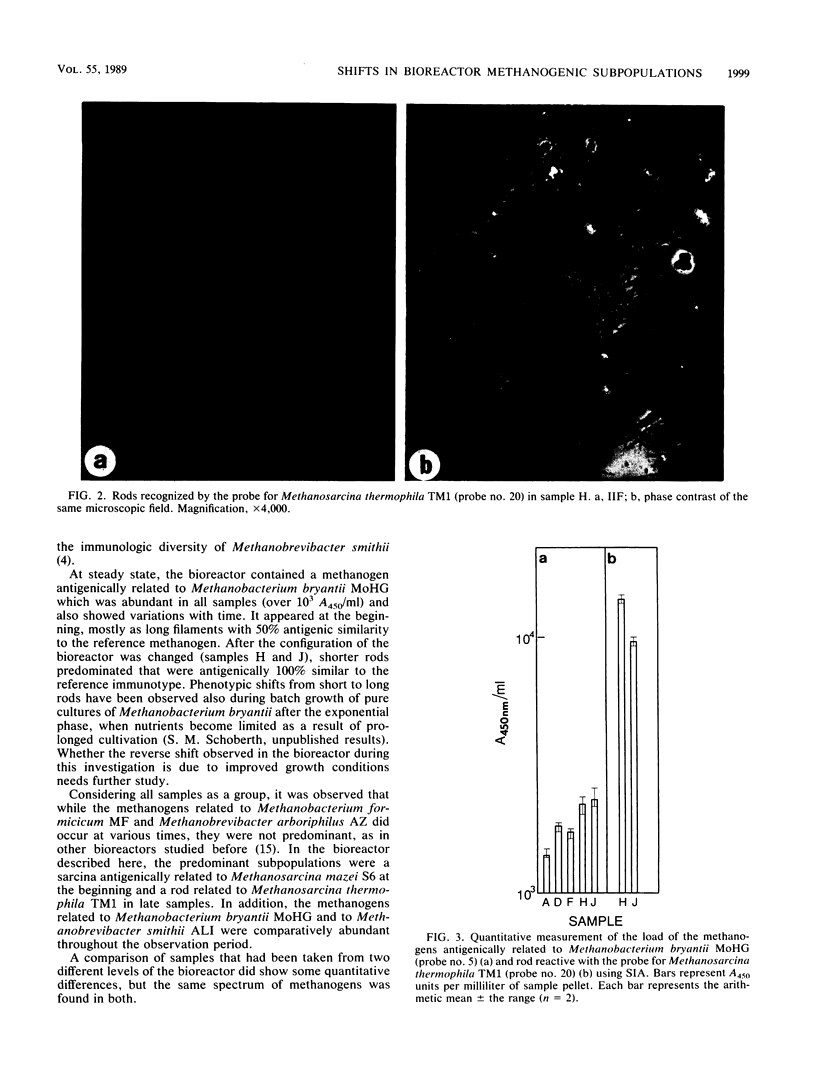
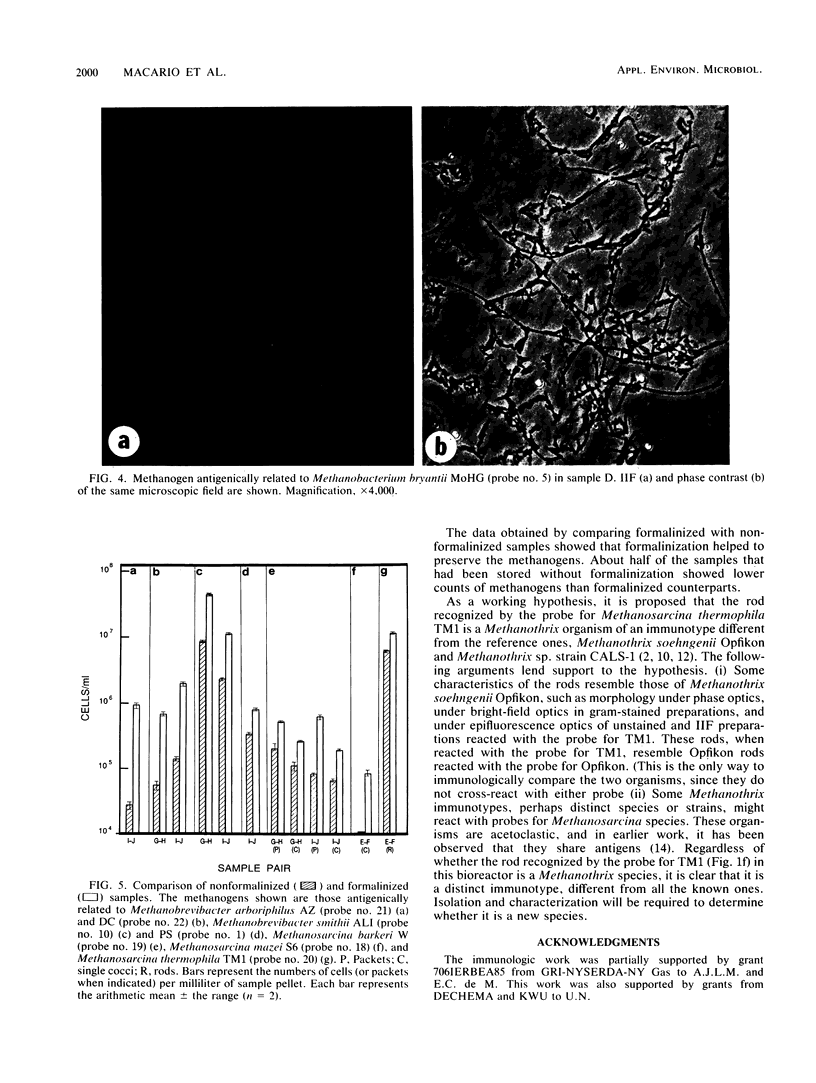

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chartrain M., Zeikus J. G. Microbial ecophysiology of whey biomethanation: characterization of bacterial trophic populations and prevalent species in continuous culture. Appl Environ Microbiol. 1986 Jan;51(1):188–196. doi: 10.1128/aem.51.1.188-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M., Smith P. H., White D. C. Examination of thermophilic methane-producing digesters by analysis of bacterial lipids. Appl Environ Microbiol. 1985 Dec;50(6):1428–1433. doi: 10.1128/aem.50.6.1428-1433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. A., Conway de Macario E., Williams R. S., Macario A. J. Direct characterization of methanogens in two high-rate anaerobic biological reactors. Appl Environ Microbiol. 1988 Mar;54(3):693–698. doi: 10.1128/aem.54.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E. Antigenic distinctiveness, heterogeneity, and relationships of Methanothrix spp. J Bacteriol. 1987 Sep;169(9):4099–4103. doi: 10.1128/jb.169.9.4099-4103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E. Quantitative immunologic analysis of the methanogenic flora of digestors reveals a considerable diversity. Appl Environ Microbiol. 1988 Jan;54(1):79–86. doi: 10.1128/aem.54.1.79-86.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Cardwell S. C., Anguish T., Lee M., Koch M. Methanogenesis in a Thermophilic (58 degrees C) Anaerobic Digestor: Methanothrix sp. as an Important Aceticlastic Methanogen. Appl Environ Microbiol. 1984 Apr;47(4):796–807. doi: 10.1128/aem.47.4.796-807.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]