Abstract
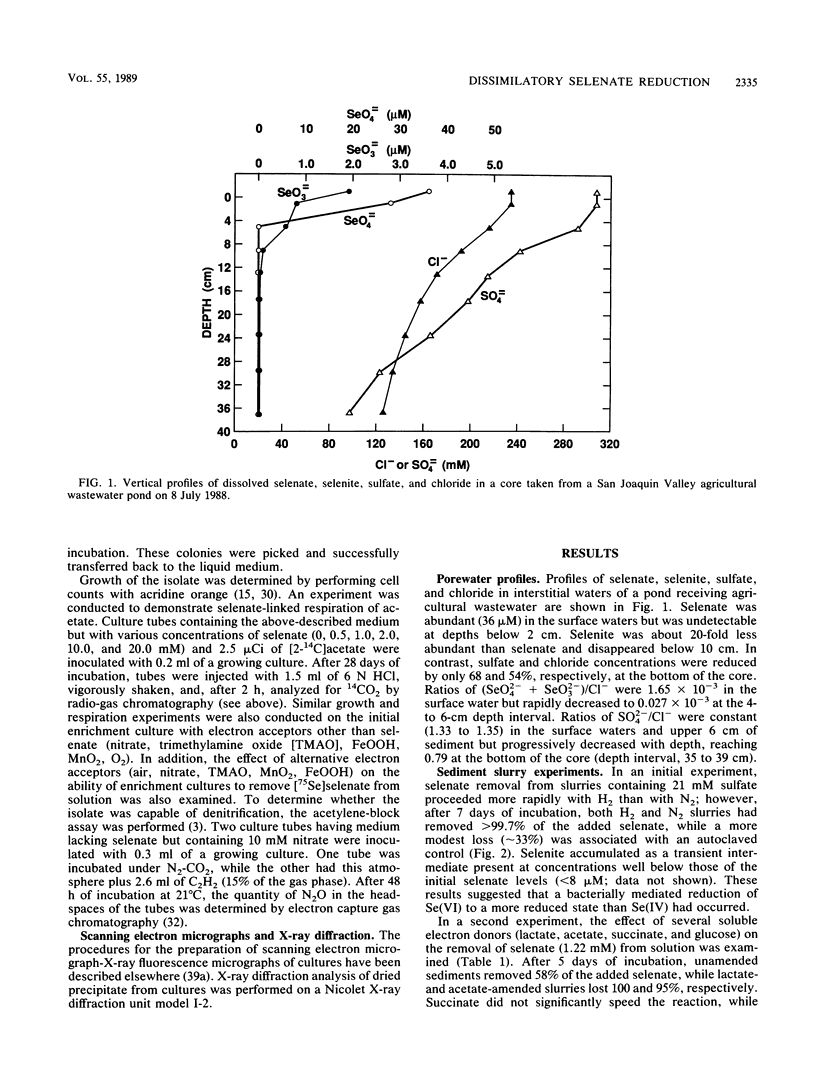
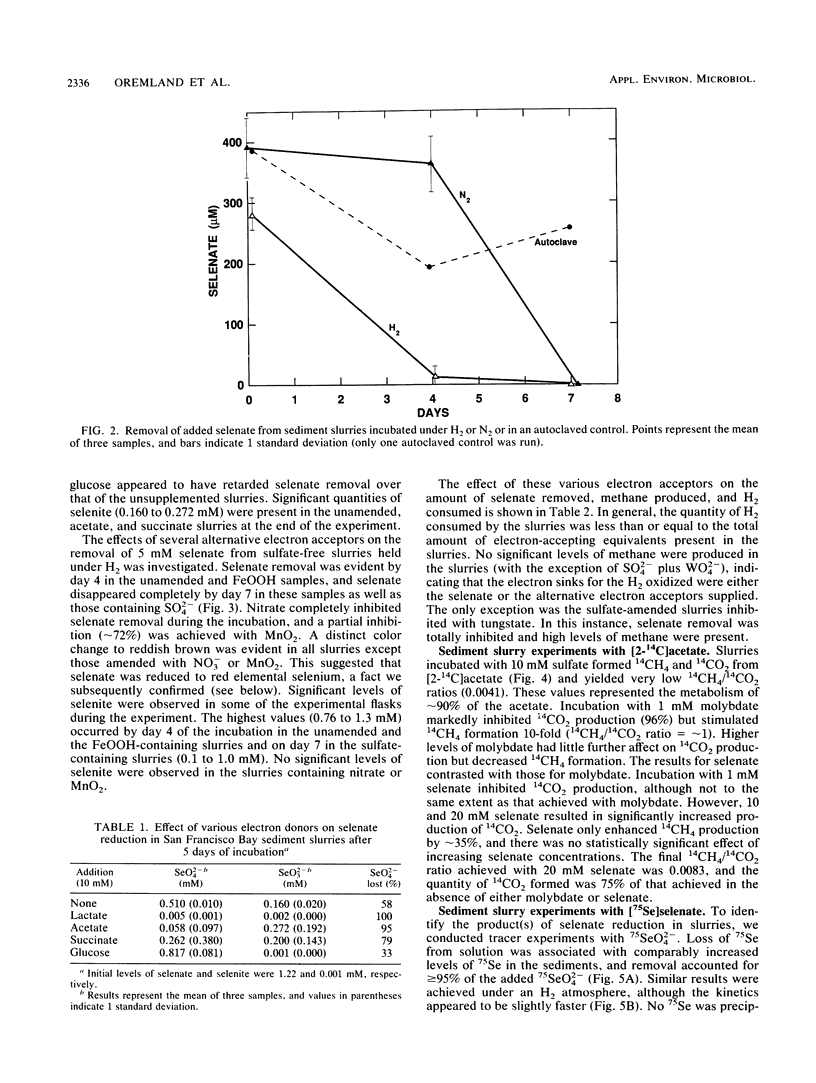
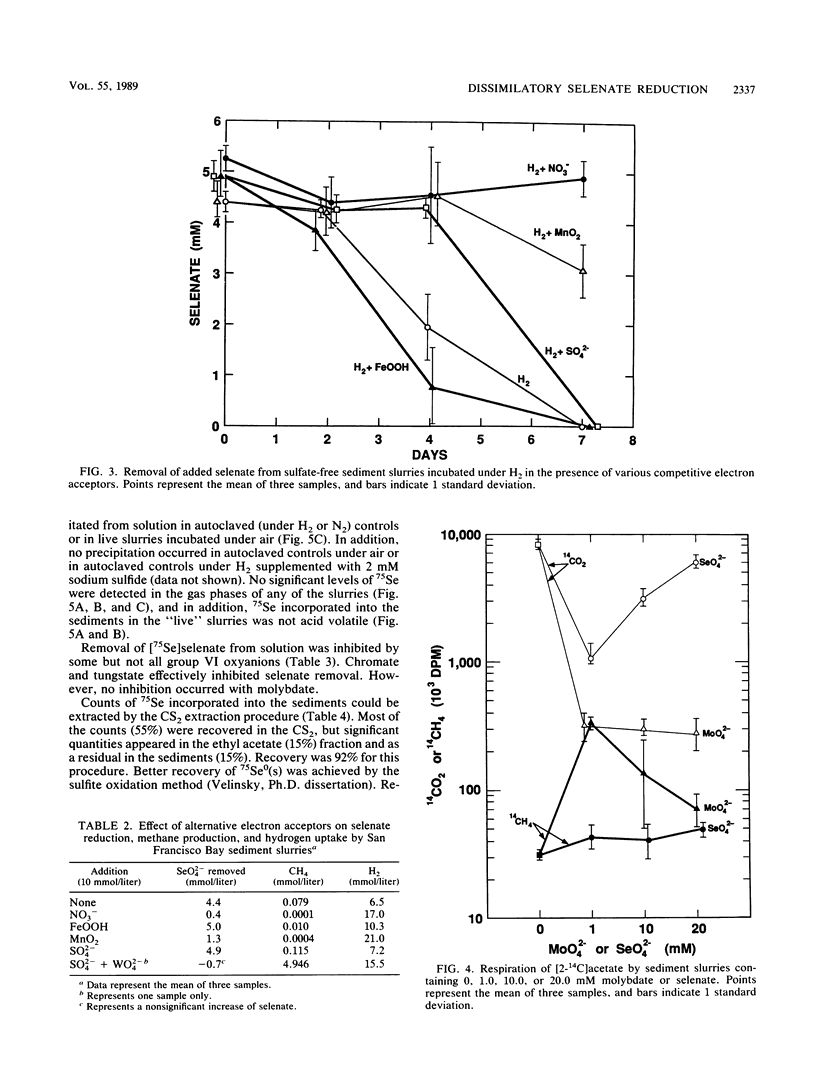
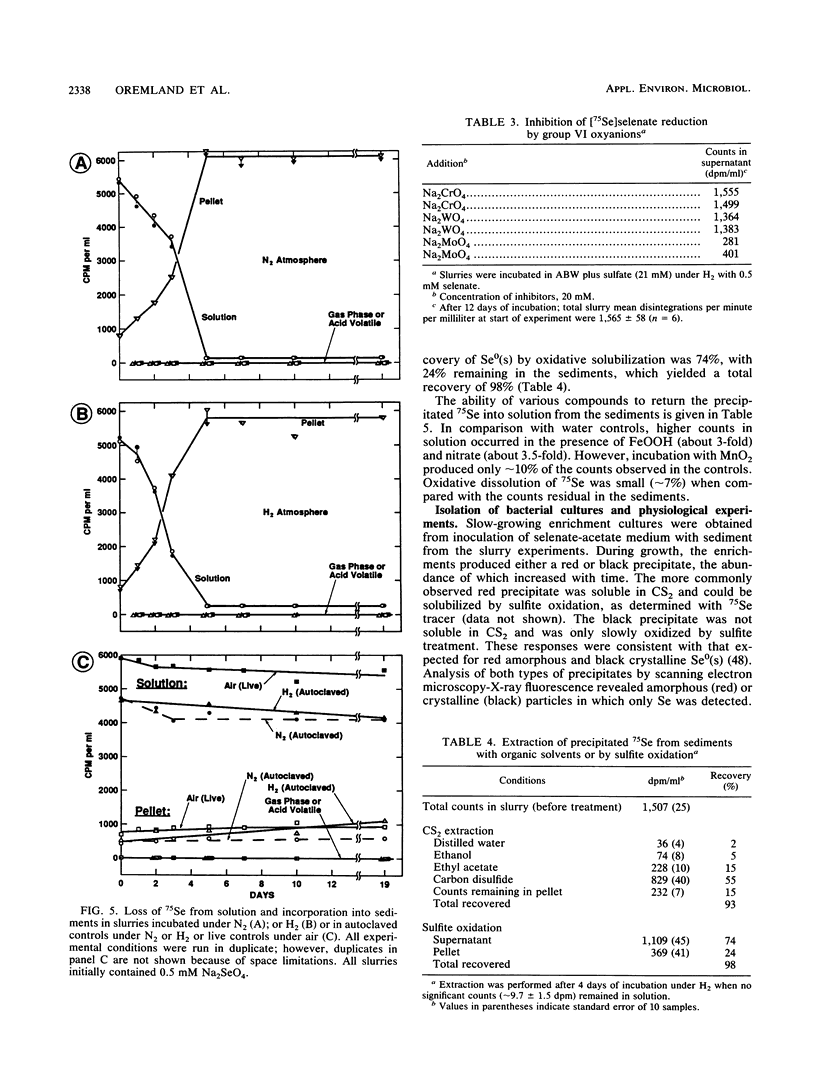
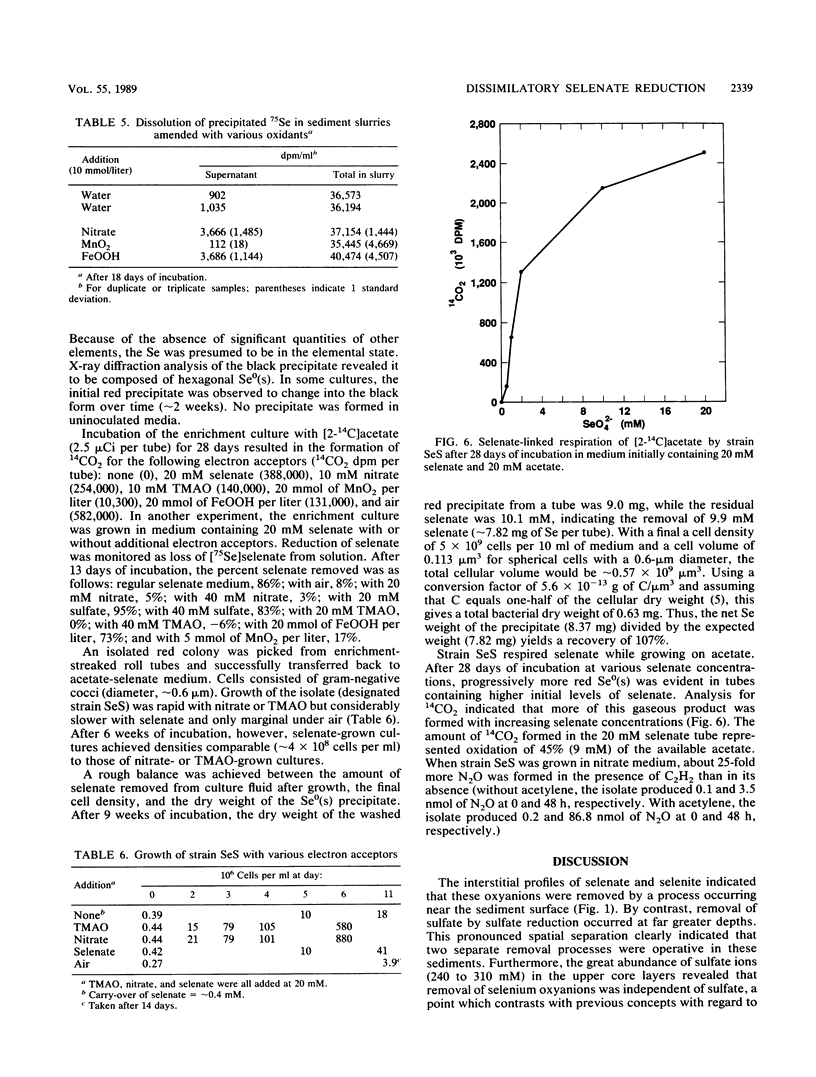
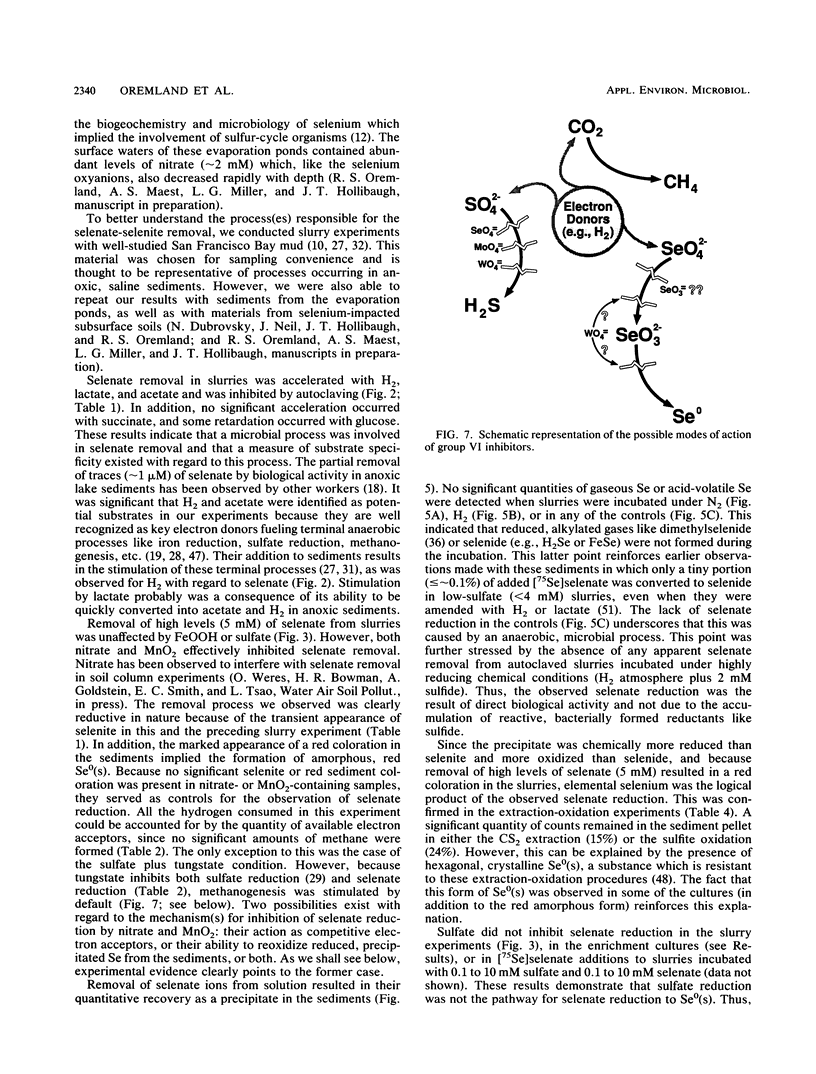
Interstitial water profiles of SeO42−, SeO32−, SO42−, and Cl− in anoxic sediments indicated removal of the seleno-oxyanions by a near-surface process unrelated to sulfate reduction. In sediment slurry experiments, a complete reductive removal of SeO42− occurred under anaerobic conditions, was more rapid with H2 or acetate, and was inhibited by O2, NO3−, MnO2, or autoclaving but not by SO42− or FeOOH. Oxidation of acetate in sediments could be coupled to selenate but not to molybdate. Reduction of selenate to elemental selenium was determined to be the mechanism for loss from solution. Selenate reduction was inhibited by tungstate and chromate but not by molybdate. A small quantity of the elemental selenium precipitated into sediments from solution could be resolublized by oxidation with either nitrate or FeOOH, but not with MnO2. A bacterium isolated from estuarine sediments demonstrated selenate-dependent growth on acetate, forming elemental selenium and carbon dioxide as respiratory end products. These results indicate that dissimilatory selenate reduction to elemental selenium is the major sink for selenium oxyanions in anoxic sediments. In addition, they suggest application as a treatment process for removing selenium oxyanions from wastewaters and also offer an explanation for the presence of selenite in oxic waters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alldredge A. L., Cohen Y. Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science. 1987 Feb 6;235(4789):689–691. doi: 10.1126/science.235.4789.689. [DOI] [PubMed] [Google Scholar]
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Dimethyl sulfoxide reductase activity by anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jun;162(3):1151–1155. doi: 10.1128/jb.162.3.1151-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G. Bacterial biovolume and biomass estimations. Appl Environ Microbiol. 1985 Jun;49(6):1488–1493. doi: 10.1128/aem.49.6.1488-1493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Shrift A. Assimilation of selenate and selenite by Salmonella typhimurium. Can J Microbiol. 1980 Jun;26(6):671–675. doi: 10.1139/m80-117. [DOI] [PubMed] [Google Scholar]
- Burton G. A., Jr, Giddings T. H., DeBrine P., Fall R. High incidence of selenite-resistant bacteria from a site polluted with selenium. Appl Environ Microbiol. 1987 Jan;53(1):185–188. doi: 10.1128/aem.53.1.185-188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran J. W., Alexander M. Microbial transformations of selenium. Appl Environ Microbiol. 1977 Jan;33(1):31–37. doi: 10.1128/aem.33.1.31-37.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblow-Kull C., Shrift A., Gherna R. L. Aerobic, Selenium-Utilizing Bacillus Isolated from Seeds of Astragalus crotalariae. Appl Environ Microbiol. 1982 Sep;44(3):737–743. doi: 10.1128/aem.44.3.737-743.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987 Nov;53(11):2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiers D. T., Wichlacz P. L., Thompson D. L., Bruhn D. F. Selenate reduction by bacteria from a selenium-rich environment. Appl Environ Microbiol. 1988 Oct;54(10):2591–2593. doi: 10.1128/aem.54.10.2591-2593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready R. G., Campbell J. N., Payne J. I. Selenite reduction by Salmonella heidelberg. Can J Microbiol. 1966 Aug;12(4):703–714. doi: 10.1139/m66-097. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Kiene R. P., Mathrani I., Whiticar M. J., Boone D. R. Description of an estuarine methylotrophic methanogen which grows on dimethyl sulfide. Appl Environ Microbiol. 1989 Apr;55(4):994–1002. doi: 10.1128/aem.55.4.994-1002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Microbial formation of ethane in anoxic estuarine sediments. Appl Environ Microbiol. 1981 Jul;42(1):122–129. doi: 10.1128/aem.42.1.122-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Zehr J. P. Formation of methane and carbon dioxide from dimethylselenide in anoxic sediments and by a methanogenic bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1031–1036. doi: 10.1128/aem.52.5.1031-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R. Competitive and noncompetitive inhibitors of bacterial sulphate reduction. J Gen Microbiol. 1952 Feb;6(1-2):128–142. doi: 10.1099/00221287-6-1-2-128. [DOI] [PubMed] [Google Scholar]
- Reamer D. C., Zoller W. H. Selenium biomethylation products from soil and sewage sludge. Science. 1980 May 2;208(4443):500–502. doi: 10.1126/science.208.4443.500. [DOI] [PubMed] [Google Scholar]
- SHRIFT A. A SELENIUM CYCLE IN NATURE? Nature. 1964 Mar 28;201:1304–1305. doi: 10.1038/2011304a0. [DOI] [PubMed] [Google Scholar]
- SHRIFT A., KELLY E. Adaptation of Escherichia coli to selenate. Nature. 1962 Aug 18;195:732–733. doi: 10.1038/195732a0. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., MacIsaac D. P., Bishop R. E., Bilous P. T. Purification and properties of Escherichia coli dimethyl sulfoxide reductase, an iron-sulfur molybdoenzyme with broad substrate specificity. J Bacteriol. 1988 Apr;170(4):1505–1510. doi: 10.1128/jb.170.4.1505-1510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J. P., Oremland R. S. Reduction of selenate to selenide by sulfate-respiring bacteria: experiments with cell suspensions and estuarine sediments. Appl Environ Microbiol. 1987 Jun;53(6):1365–1369. doi: 10.1128/aem.53.6.1365-1369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


