Abstract
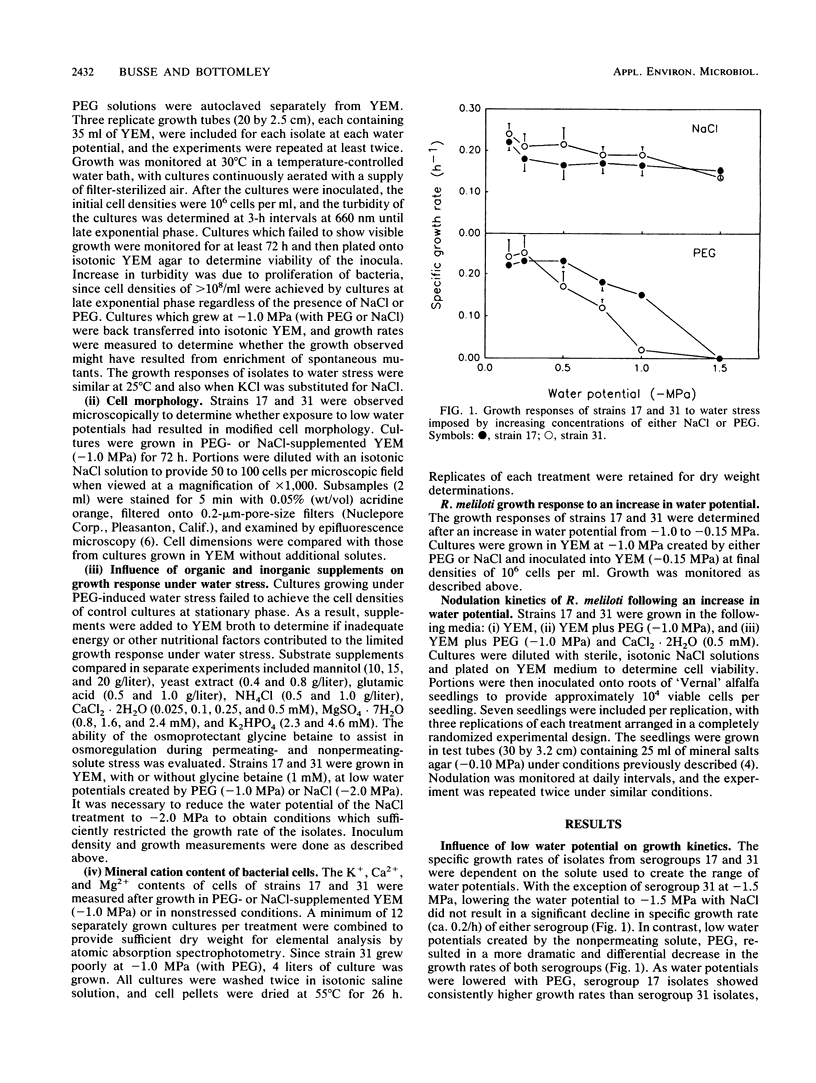
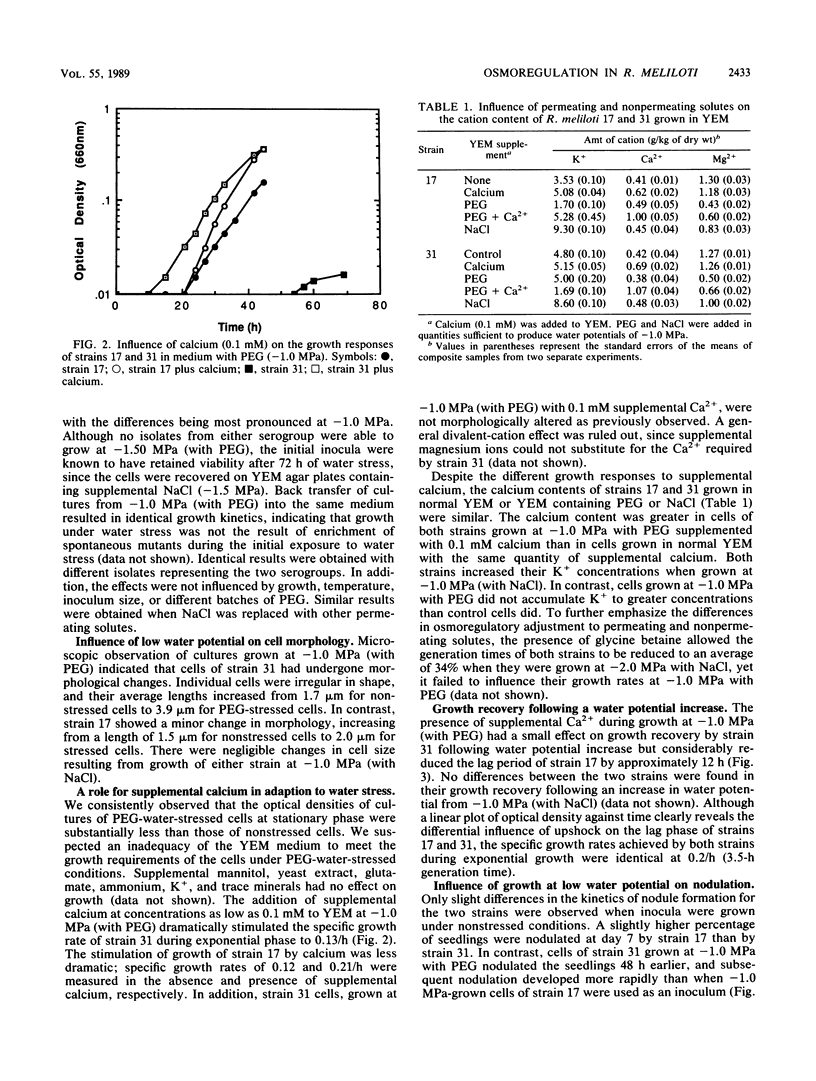
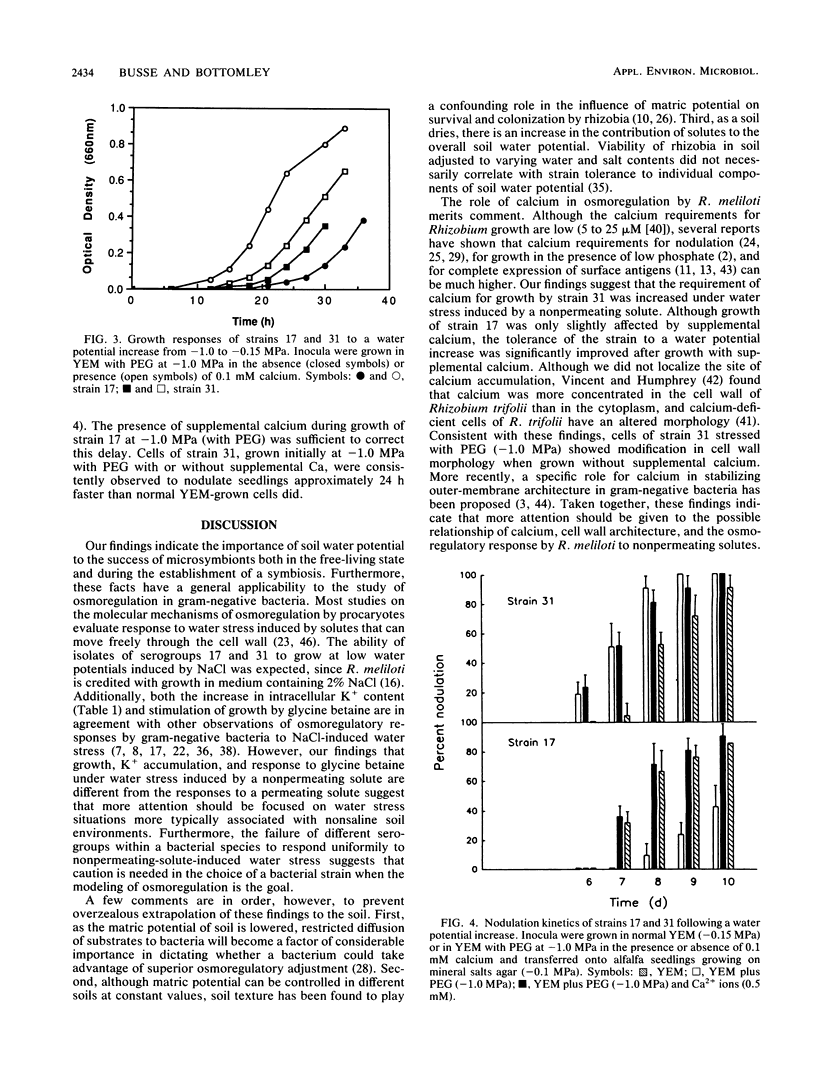
Isolates of Rhizobium meliloti, representing antigenically distinct indigenous serogroups 31 and 17, were grown in yeast extract-mannitol broth (YEM) containing NaCl or polyethylene glycol (PEG) to provide external water potentials ranging from −0.15 to −1.5 MPa. Several differences were found between representatives of the two groups in their abilities to adapt to water stress induced by the nonpermeating solute PEG. At potentials below −0.5 MPa, strain 31 had a lower specific growth rate than strain 17 and an irregular cell morphology. In contrast, neither growth nor cell morphology of either strain was affected significantly over the same range of water potentials created by a permeating solute, NaCl. Despite the superior growth of strain 17 at the low water potentials imposed by PEG, upshock of water-stressed cells (−1.0 MPa; PEG) into normal YEM (−0.15 MPa) resulted in a faster recovery of growth by strain 31 than by strain 17. Different responses of the two strains to a water potential increase were also revealed in nodulation studies. Strain 31 required significantly fewer days to nodulate alfalfa than strain 17 did when the strains were transferred from YEM with PEG at −1.0 MPa onto the roots of alfalfa seedlings in plant growth medium (−0.1 MPa). The addition of supplemental calcium (0.1 mM) to growth medium with PEG (−1.0 MPa) reduced the differences between strains in their responses to water stress. The severe growth restriction and morphological abnormalities shown by strain 31 were corrected, and the prolonged recovery time shown by water-stressed cells (−1.0 MPa; PEG) of strain 17 upon transfer to normal YEM was shortened. The latter strain also nodulated earlier and more rapidly after growth in PEG medium at −1.0 MPa in the presence of supplemental calcium ions. These results indicate that the efficacy of osmoregulation can vary among strains of the same species and that the mechanism of osmoregulation may differ depending on the nature of the water stress.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenguer J., Faraldo M. L., de Pedro M. A. Ca2+-stabilized oligomeric protein complexes are major components of the cell envelope of "Thermus thermophilus" HB8. J Bacteriol. 1988 Jun;170(6):2441–2447. doi: 10.1128/jb.170.6.2441-2447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Autecology in Rhizospheres and Nodulating Behavior of Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1014–1019. doi: 10.1128/aem.52.5.1014-1019.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. In situ measurement of plant water potentials by equilibration with microdroplets of polyethylene glycol 8000. Plant Physiol. 1985 Sep;79(1):270–273. doi: 10.1104/pp.79.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuquay J. I., Bottomley P. J., Jenkins M. B. Complementary Methods for the Differentiation of Rhizobium meliloti Isolates. Appl Environ Microbiol. 1984 Apr;47(4):663–669. doi: 10.1128/aem.47.4.663-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B. A., Vincent J. M. The effect of calcium nutrition on the production of diffusible antigens by Rhizobium trifolii. J Gen Microbiol. 1965 Oct;41(1):109–118. doi: 10.1099/00221287-41-1-109. [DOI] [PubMed] [Google Scholar]
- Jovanovich S. B., Martinell M., Record M. T., Jr, Burgess R. R. Rapid response to osmotic upshift by osmoregulated genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):534–539. doi: 10.1128/jb.170.2.534-539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M. R., Eckard A. N. Evaluation of water stress control with polyethylene glycols by analysis of guttation. Plant Physiol. 1971 Apr;47(4):453–456. doi: 10.1104/pp.47.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B., Dowdle S., Sadowsky M. J. Competition of Rhizobium japonicum Strains in Early Stages of Soybean Nodulation. Appl Environ Microbiol. 1983 Oct;46(4):870–873. doi: 10.1128/aem.46.4.870-873.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Lowther W. L., Loneragan J. F. Calcium and Nodulation in Subterranean Clover (Trifolium subterraneum L.). Plant Physiol. 1968 Sep;43(9):1362–1366. doi: 10.1104/pp.43.9.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton P. W., El Swaify S. A., Bohlool B. B. Effect of salinity on Rhizobium growth and survival. Appl Environ Microbiol. 1982 Oct;44(4):884–890. doi: 10.1128/aem.44.4.884-890.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuter A. A. Water potential of aqueous polyethylene glycol. Plant Physiol. 1981 Jan;67(1):64–67. doi: 10.1104/pp.67.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland L., Cairney J., Elmore M. J., Booth I. R., Higgins C. F. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986 Nov;168(2):805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M., HUMPHREY B. A. PARTITION OF DIVALENT CATIONS BETWEEN BACTERIAL WALL AND CELL CONTENTS. Nature. 1963 Jul 13;199:149–151. doi: 10.1038/199149a0. [DOI] [PubMed] [Google Scholar]
- VINCENT J. M. Influence of calcium and magnesium on the growth of rhizobium. J Gen Microbiol. 1962 Sep;28:653–663. doi: 10.1099/00221287-28-4-653. [DOI] [PubMed] [Google Scholar]
- Vincent J. M., Humphrey B. A. Modification of the antigenic surface of Rhizobium trifolii by a deficiency of calcium. J Gen Microbiol. 1968 Dec;54(3):397–405. doi: 10.1099/00221287-54-3-397. [DOI] [PubMed] [Google Scholar]
- Wee S., Wilkinson B. J. Increased outer membrane ornithine-containing lipid and lysozyme penetrability of Paracoccus denitrificans grown in a complex medium deficient in divalent cations. J Bacteriol. 1988 Jul;170(7):3283–3286. doi: 10.1128/jb.170.7.3283-3286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


