Abstract
Purpose:
To characterize the effect of postural IOP elevation and pharmacological IOP lowering on retinal ganglion cell (RGC) function in the DBA/2J mouse model of glaucoma.
Methods:
Four groups of DBA/2J mice (3 month old, n=7; 5 month old, n=7, 10 month old, n=7, 11 month old, n=8) were anesthetized with intraperitoneal injections (0.6 ml/Kg) of a mixture of Ketamine (42.8 mg/ml), Xylazine (8.5 mg/ml) and Acepromazine (1.4 mg/ml). IOP and Pattern Electroretinogram (PERG) were sequentially measured with mice in horizontal position (0°), 60° head-down, and again at 0°. IOP and PERG were also measured before and after intraperitoneal mannitol 25% (2.5 g/Kg) administration with mice in horizontal position.
Results:
Head-down position induced reversible IOP elevations of 32-38% in all age groups (P<0.01), and age-dependent reductions of PERG amplitude (3 months: +3%; 5 months: − 47%, P<0.01; 10 months: −65%, P<0.01). Mannitol administration in 11 month-old mice resulted in IOP lowering of about 38% (P<0.01) and PERG amplitude improvement of about 83% (P<0.001). IOP and PERG amplitude changes were inversely correlated (10 months head-down r2 = 0.58, P<0.001; 10 month-old mannitol r2 = 0.41, P<0.001). For all conditions, the light-adapted flash ERG was unaltered.
Conclusions:
In the DBA/2J mouse, RGC susceptibility to artificial IOP elevation increases with age. Abnormal RGC function in older mice may be improved with IOP lowering. Evaluation of PERG changes in response to artificial IOP modulation may represent a powerful tool to non-invasively assess RGC susceptibility to IOP insult in genetically distinct mouse models of glaucoma.
Keywords: Retinal ganglion cells, glaucoma, pattern electroretinogram, mouse, DBA/2J, cell death
Introduction
Mouse models with genetic alterations relevant to glaucoma are receiving increasing attention to better understand the complex nature of the disease and design specific treatments to prevent death of retinal ganglion cells (RGCs) and their axons.1-4
The DBA/2J mouse is a well established model of spontaneous glaucoma. Recessive mutations in two genes, Gpnmb and Tyrp1, cause iris atrophy and pigment dispersion.5 The iris disease is apparent at 6 months and progresses with age, resulting in elevated intraocular pressure (IOP), loss of RGCs and optic nerve axons, and optic disk excavation.6 Young (2-3 month-old) DBA/2J mice have normal IOP and normal histological appearance of RGCs and optic nerves. By 8-9 months of age RGCs show signs of apoptotic death.7, 8Axonal damage in the optic nerves is apparent in about 50% of eyes by 10 to 11 months of age, and in about 90% of eyes by 18 months.6
Surviving RGCs may not be functional. Functional events associated to IOP elevation and RGC progressive degeneration in DBA/2J mice need to be elucidated. RGC function in mice can be evaluated by means of the Pattern Electroretinogram (PERG).9-12 The non invasive nature of the PERG allows serial recordings as a function of changing conditions (e.g., age, IOP levels). Using PERG, we have been able to characterize the natural history of RGC dysfunction and its association with IOP in a 12-month longitudinal study of BDA/2J mice.13 On average, the IOP increased from 14 mm Hg to 18 mm Hg between 2 and 6 months and then more steeply to level off by 11 months at a value of about 28 mm Hg.13 After 3 months, the PERG amplitude decreased progressively with age to approach the noise level at about 10-11 months.13 The time-course of IOP elevation and PERG amplitude reduction were closely associated. Histological analysis of eyes with abolished PERG showed that the retinal nerve fiber layer (RNFL) had lost only about 40% of the normal thickness, and the cone-flash ERG did not significantly change.13 Taken together, these results indicated that DBA/2J mice develop a progressive functional damage in the inner retina (abnormal PERG) but not in the outer retina (normal flash-ERG) that seems to precede anatomical damage of the optic nerve (relatively spared RNFL).
Recently, Aihara and coll.14 reported in NIH white Swiss mice that head-down position induces substantial elevation of IOP associated with elevation of the episcleral venous pressure (EVP). Head-down position induces very similar IOP and EVP changes in human.(e.g.,15) In the present study we tested the hypothesis that IOP elevation in the head-down position causes reduction of PERG amplitude in susceptible eyes of older DBA/2J mice. We also tested the hypothesis that impaired RGC function in older DBA/2J mice may be improved by pharmacologically reducing IOP with mannitol.16 Preliminary results of this study have been presented at ICER, 2006 17 and ARVO, 2007.18
Methods
Animals and husbandry
A total of 29 DBA/2J mice of different ages — 3 month old, n=7 (7 eyes); 5 month old, n=7 (7 eyes), 10 month old, n=7 (7 eyes), 11 months, n=8, (16 eyes) — obtained by Jackson Labs, Bar Harbor, Maine were tested. Mice were maintained in a cyclic light environment (12-h 50 lux, 12-h dark) and fed ad libitum. For both IOP and PERG measurements, mice were weighed and anesthetized with intraperitoneal injections (0.5-0.7 ml/Kg) of a mixture of Ketamine, 42.8 mg/ml, Xylazine, 8.6 mg/ml and Acepromazine, 1.4 mg/ml. All procedures were performed in compliance with the ARVO statement for use of animals in ophthalmic and vision research. The experimental protocol was approved by the Animal Care and Use Committee of the University of Miami.
IOP measurement
IOP was measured with an induction/impact tonometer (Tonolab Colonial Medical Supply, Franconia, NH).19 The tonometer was fixed in a vertical position to a support stand by means of clamps. Anesthetized mice were placed on an adjustable stand and the tail restrained with adhesive tape. The probe tip was aligned with the optical axis of the eye at 1-2 mm distance using a magnifier lamp. Five consecutive IOP readings were averaged. Importantly, the impact of the Tonolab probe on the cornea is minimal, such as local corneal anesthesia is not necessary.20 In a previous longitudinal study over one year 13 we did not find obvious corneal damage at biomicroscopic examination. IOP readings obtained with Tonolab have been proven to be accurate and reproducible in different mouse strains including DBA/2J.13, 20
PERG recording
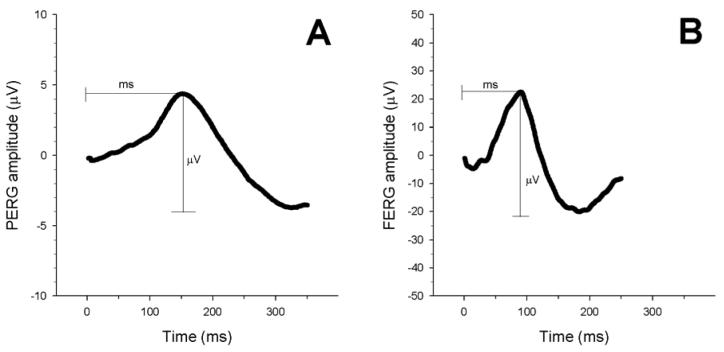
Detailed description of the PERG technique is reported elsewhere.11, 12 In brief, anesthetized mice were gently restrained using a mouth bite bar and a nose holder that allowed unobstructed vision,11, 21 and kept at a constant body temperature of 37.0 ° C using a feedback-controlled heating pad. The body of the mouse could be oriented horizontally as well as 60 deg head-down on an adjustable platform by holding the tip of the tail with adhesive tape. The eyes of anesthetized mice were typically wide open and in a stable position, with undilated pupils pointing laterally and upwardly. The ERG electrode (0.25 mm diameter silver wire configured to a semi-circular loop of 2 mm radius) was placed on the corneal surface by means of a micromanipulator and positioned in such a way as to encircle the undilated pupil without limiting the field of view. Reference and ground electrodes were stainless steel needles inserted under the skin the scalp and tail, respectively. A small drop of balanced saline maintained the cornea and lens in excellent conditions for the duration of recording. A visual stimulus of contrast-reversing bars (field area: 50 × 58 deg; mean luminance 50 cd/m2; spatial frequency: 0.05 cycles/deg, contrast: 98%; temporal frequency: 1Hz) was aligned with the projection of the undilated pupil at 20 cm distance. Eyes were not refracted for the viewing distance since the mouse eye has a large depth of focus due to the pinhole pupil.22, 23 Retinal signals were amplified (10,000 fold) and band-pass filtered (1-30 Hz). Three consecutive responses to 600 contrast reversals each were recorded. The responses were superimposed to check for consistency and then averaged. The PERG is a light-adapted response. To have a corresponding index of outer retina function, a light-adapted ERG (FERG) was also recorded with undilated pupils in response to strobe flashes of (20 cd/m2/s) superimposed on a steady background light (12 cd/m2) and presented within a Ganzfeld bowl. Under these conditions rod activity is largely suppressed while cone activity is minimally suppressed.24 Averaged PERGs and FERGs were automatically analyzed to evaluate the major positive and negative waves (Figure 1). For both PERG and FERG, peak-to-trough response amplitude was automatically evaluated on the major positive-negative complex, and the latency was the time-to-peak of the major positive deflection. Separate analysis of positive and negative components of responses was not systematically performed since unambiguous recognition of these components was not possible in older mice with reduced PERG signal.
Figure 1.
Examples of PERG (A) and FERG (B) recorded in DBA/2J mice aged 3 months. For both PERG and FERG, the amplitude was measured from the positive peak to the negative trough. The latency was evaluated from the stimulus onset (contrast reversal for the PERG, strobe flash for the FERG) to the peak of the positive wave. Note that the PERG has smaller amplitude and longer latency compared to the FERG.
Pupil size
Anesthesia may cause pupil dilation, 25 and older DBA/2J may have slight pupil dilation or deformation due to the pathologic iris condition.5 The size of the pupil was continuously checked during sequential IOP measurements using a magnifier lamp. Pupil size was also checked with a microscope (25x) at the beginning and at the end of the PERG recording together with assessment of correct positioning of ERG electrodes. A four-point grading system was used to approximately describe the pupil size: small, slightly dilated, moderately dilated, dilated. All mice had small or slightly dilated baseline pupils; the pupil size did not appreciably change after either head-down posture or mannitol administration.
Experiment #1 — IOP elevation
After two baseline IOP measurements performed with the animal resting in a horizontal position, the adjustable platform was tilted in such a way as to put mice in a head-down (60 deg) position for 30 minutes and then repositioned horizontally for at least 20 minutes (recovery). Sequential IOP measurements (1 to 5 minutes apart) were obtained over the whole tilted-recovery period. The adjustable platform was then inserted in the stereotaxic holder for PERG recording. Since recording time for PERG/FERG recording was approximately 15 minutes, only tree sequential responses could be obtained during the observation period: baseline, 15 to 30 minutes after head-down position, and 15 to 30 minutes after horizontal repositioning.
Experiment #2 — IOP lowering
With the animal resting in a horizontal position, two baseline IOPs were measured. One intraperitoneal injection of mannitol 25% (2.5 g/Kg; Hospira, Inc. Lake Forest, USA) was then administered, and IOP was measured approximately every 5 minutes over one hour. Mice were then allowed to recover from anesthesia and returned to their cages. One week later, IOP was again measured with the animal resting in a horizontal position as a control that IOP recovered initial baseline values. Mice were then inserted in the stereotaxic holder for PERG recording in horizontal position. After a baseline PERG/FERG were recorded, one intraperitoneal injection of mannitol 25% was administered and PERG/FERG were recorded 20-60 minutes later. No further IOP measures were taken.
Time-course of IOP changes
In most eyes of each age group, the number of data points was sufficient to mathematically fit the time-course of IOP rise and decay. Data were well fitted with exponential rise and decay functions [exponential rise: IOP(t) = pIOP + dIOP · (1− e−x / tau) ; exponential decay: IOP(t) = pIOP + dIOP · (e −x / tau)], where pIOP represents the plateau IOP, dIOP represents the delta IOP, and tau represents the time constant of IOP change. The time constant is the time required to change the baseline value by a 1/e (63%) factor.
Results
Experiment #1 — Effect of IOP increase
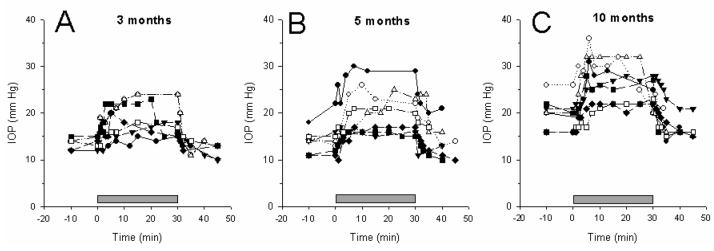
Figure 2 displays the time-course of IOP changes measured in the left eyes of 21 mice of different ages under baseline condition (horizontal), tilted (60 deg head-down), and recovery (horizontal). Upon head-down positioning, the IOP progressively increased in all eyes and reached a relatively stable value. Upon horizontal repositioning, the IOP progressively returned to baseline values. The pupil size did not appreciably change during the entire observation period. The time-courses of IOP rise and decay were symmetric, with a time constant on the order of 4 minutes on average (IOP rise tau = 3.6 ± 2.2; IOP decay tau = 4.6 ± 3.3).
Figure 2.
Time course of IOP changes during head down (60 deg) body tilting (grey bar over the x-axis) in DBA/2J mice of different ages (A: 3 months; B: 5 months; C: 10 months). Different symbols correspond to different mice.
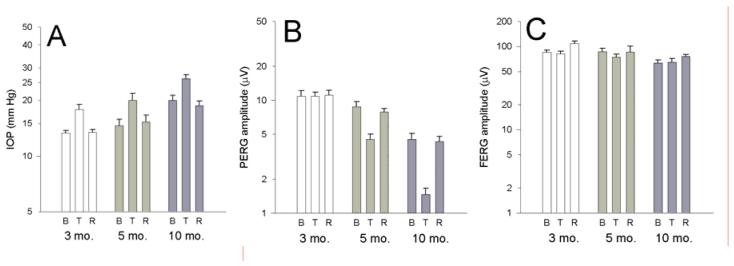
Average PERG amplitudes recorded in horizontal position (baseline), 10-30 minutes after head-down position (tilted), and 10-30 minutes after repositioning mice in horizontal position (recovery) are displayed together with corresponding IOP and FERG amplitude (Figure 3). Data are plotted on log scales to normalize differences with respect to baseline conditions as well as relative changes of PERG amplitude with respect to IOP changes. The mean baseline IOP increased with increasing age (3 months: 13.2 ± 1.1 mm Hg; 5 months: 14.6 ± 3.2 mm Hg; 10 months: 20.0 ± 3.4 mm Hg, ANOVA, P<0.01). The increase in baseline IOP with age was significant between 10 month-old mice and 3 month-old mice (P<0.001) but not between 5 month-old mice and 3 month-old mice (P=0.33). The delta IOP (tilted minus horizontal baseline) was on the order of 5 mm Hg and the relative change (tilted /horizontal baseline*100) did not change significantly with age (3 months: 33.8 ± 13.9%; 5 months: 37.8 ± 17.9 %; 10 months: 32.4 ± 10.7 %, ANOVA, P=0.77). The mean baseline PERG amplitude decreased with increasing age (3 months: 10.8 ± 3.5 μV; 5 months: 8.7 ± 2.4 μV; 10 months: 4.5 ± 1.5 μV, ANOVA, P<0.01). The decrease in baseline PERG amplitude with age was significant between 10 month-old mice and 3 month-old mice (P<0.001) but not between 5 month-old mice and 3 month-old mice (P=0.28). Differently from IOP, the % PERG amplitude change (tilted/horizontal baseline*100) was strongly dependent on age (3 months: 3.4 ± 16.5 %; 5 months: − 46.8 ± 15.4 %; 10 months: − 65 .4 ± 14.0 %, ANOVA, P<0.001). In 3 month-old mice the PERG amplitude change was not significantly different from zero, whereas in both 5 month-old mice and 10 month-old mice PERG amplitude reductions in head-down position were highly significant (P<0.001). Relative PERG amplitude reductions in 10 month-old mice were significantly (P<0.05) larger than those of 5 month-old mice. Note, by comparing panel A with panel B, the age-dependent disproportion between logarithmic differences of IOP and PERG amplitude. In all mice, the PERG amplitude fully recovered baseline values when mice were tested again in horizontal position. The head-down position did not cause significant changes in FERG amplitude. The peak latency of both PERG and FERG did not change significantly across conditions (not shown in figures).
Figure 3.
A: Average (±SEM) values for IOP (A), PERG amplitude (B) and FERG amplitude (C) in DBA/2J mice of different ages measured in horizontal baseline condition (B), during head-down body tilting (T), and during recovery (R) in horizontal position. Data are plotted on log scales to normalize relative changes.
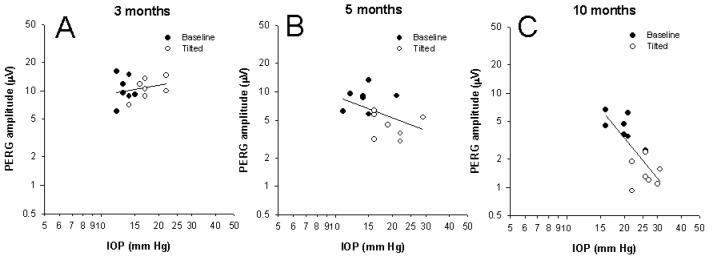
Mean PERG amplitudes recorded in baseline and tilted positions have been plotted against corresponding mean IOP values, and data fitted with linear regressions (Figure 4). The correlation between PERG amplitude and IOP increases with increasing age of mice (3 month-old: r2 = 0.04, P=0.53; 5 month-old: r2 = 0.17, P=0.14; 10 month-old: r2 = 0.58, P<0.001).
Figure 4.
Changes in PERG amplitude and IOP after 60 deg head-down body tilting in DBA/2J mice of different ages (A: 3 months; B: 5 months; C: 10 months). Data have been fitted with linear regression lines. Closed symbols: baseline condition; open symbols: tilted condition.
Experiment #2 —Effect of IOP decrease
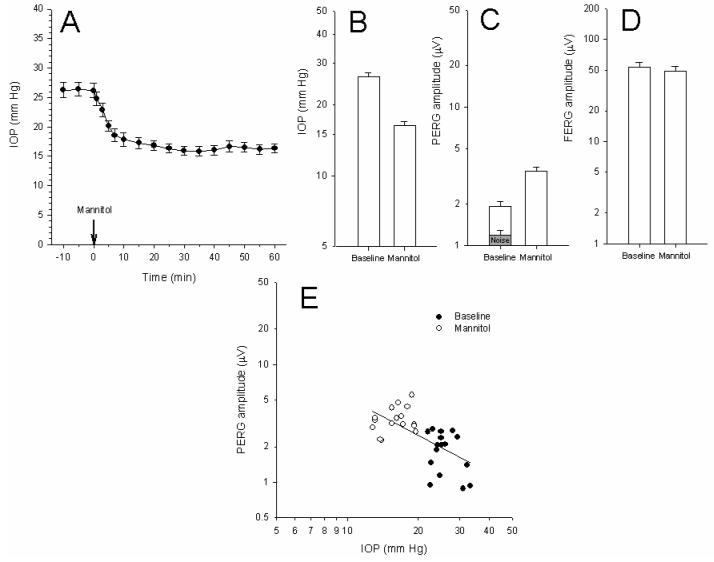
Figure 5A displays the time-course of IOP changes measured in 8 mice (16 eyes) aged 11 months in horizontal position after intraperitoneal injection of 25% mannitol. The time-course of IOP decay had a time constant on the order of 5-6 minutes (average tau = 5.5 ± 3.5). The pupil size did not appreciably change during the entire observation period. As shown in Figure 5 B the untreated IOP was 26.3 ± 4 mm Hg on average. Mannitol treatment consistently reduced the IOP to a relatively stable average value of 16.3 ± 2.8 mm Hg (−38%) (P<0.001) over the next 20-60 minutes. When the same mice were tested one week later, IOP had recovered baseline values (mean 26.0 ± 4.3). PERGs and FERGs were then recorded before and 20-60 minutes after mannitol treatment. The baseline PERG amplitude of these 11 month-old DBA/2J mice (Figure 5 C) was typically very low13 and close to the noise level, as measured by recording a response to a pattern stimulus of zero contrast.11 After mannitol treatment the PERG amplitude improved by about 83% (P<0.01) (Figure 5 C). Mannitol treatment had no significant effect on FERG amplitude (Figure 5 E), PERG latency, and FERG latency (not shown in figures).
Figure 5.
Effect of mannitol treatment on IOP and PERG in 11 month-old DBA/2J mice. A: Time-course of IOP after mannitol treatment. B, C, D: Mean (± SEM) IOP (B), PERG amplitude (C), and FERG amplitude (D). The grey bar in (C) represents the average (± SEM) noise level. Data are plotted on log scales to normalize relative changes. E: Changes in PERG amplitude and IOP after mannitol treatment. Data have been fitted with linear regression lines. Closed symbols: baseline condition; open symbols: mannitol treatment.
Mean PERG amplitudes recorded before and after mannitol have been plotted against corresponding mean IOP values (Figure 5E), and data fitted with a linear regression (r2 = 0.41, P<0.001).
Discussion
In agreement with previous results,14 this study shows that it is possible to induce transient and consistent elevations of IOP in Ketamine-Xylazine anesthetized DBA/2J mice of different age by positioning the animals 60 deg head-down. The amount of IOP increase (mean about 5 mm Hg) is larger than that (mean 2.6 mm Hg) reported in Ketamine-Xylazine anesthetized NIH Swiss white mice positioned 60 deg head-down.14 The reason for this difference might be due to the different technique of IOP measurement. The present data were obtained with a non-invasive rebound tonometer (Tonolab) whereas previous results 14 were obtained by cannulating the anterior chamber (AC) with a water-filled microneedle connected to a pressure transducer.26 However, baseline IOP values measured in this study (13.2 ± 1.1 mm Hg in 3 month-old mice) are similar to those measured with AC cannulation in 3 month-old NIH Swiss white mice (14.8 ±2.3 mmHg,2716.5 ± 0.6 mm Hg 14). In addition, IOP readings obtained with rebound tonometer compare very well with IOP readings obtained with AC cannulation. 20, 28 Overall, it appears more likely that the differences in posture-induced IOP elevation are due to genetic differences between DBA/2J and NIH Swiss white mice rather than different technique of IOP measurement.
Posture-induced IOP elevation is closely related to increase of the episcleral venous pressure (EVP) as experimentally demonstrated in human 29, 30 as well as mouse. 14 EVP increase in human subjects and mice is likely caused by increase in the volume of the choroidal vascular bed.14, 30, 31 The time course of choroidal engorgement upon head-down position resulting in elevated EVP is expected to be relatively brief (seconds). Our data show that the time course of IOP elevation is much longer (minutes). The gradual rise of IOP probably reflects the dynamic interaction between the rate of aqueous humor formation and the establishment of a new equilibrium IOP to compensate for the increase in afterload to aqueous outflow.(discussed in 32, 33) A similar reasoning applies for the slow decay of IOP upon horizontal repositioning. The mouse anterior chamber 34 and the turnover of aqueous humor 35 are comparable to those of human. In human subjects, postural changes also induce changes in systemic blood pressure (e.g., 36) and ophthalmic artery pressure that may have a role in IOP regulation. These measures are not available for the mouse. However, Savinova and collaborators, 200137 did not find a positive correlation between blood pressure and IOP in different mouse strains whose blood pressure differed up to 36 mm Hg.
This study shows that postural IOP elevation induces reversible, age-dependent reductions of PERG amplitude in DBA/2J mice. The baseline IOP, the amount of IOP elevation upon tilting, and the baseline PERG amplitude were similar in 3 month-old and 5 month-old mice. However, clear PERG amplitude reduction occurred in 5 month-old mice but not in 3 month-old mice. This indicates increased susceptibility of retinal neurons to IOP insult in 5 month-old mice compared to 3 month olds. In 10 month-old mice the baseline IOP was increased, and the PERG amplitude decreased, compared to 3 month olds. IOP elevation upon tilting exacerbated the pre-existing PERG amplitude deficit to larger extent compared to 5 month-old mice. In mice of all ages, posture-induced IOP elevation did not cause FERG changes, thereby excluding a generalized retinal effect and indicating that PERG changes reflect inner retina dysfunction.
This study also shows that intraperitoneally administered mannitol consistently reduced IOP by 38% on average. Both the amount of IOP reduction and the time course of IOP changes are in good agreement with previous results.16 In 11 month-old DBA/2J mice, in which the baseline PERG was close to the noise level, IOP reduction resulted in a marked improvement of PERG amplitude by about 83% on average. Altogether, results indicate that reduced PERG signal in 11 month-old DBA/2J mice results, at least in part, from reduced responsiveness of viable retinal neurons rather than lack of signal of dead neurons. Reduced responsiveness is alleviated by IOP reduction. IOP reduction does not cause FERG changes, thus indicating that PERG changes reflect selective improvement of inner retina dysfunction.
Thus, in older DBA/2J mice RGC dysfunction appears to be strongly IOP-dependent since it can be either exacerbated or alleviated, respectively, by experimental elevation or lowering of the IOP. This does not imply that IOP is the only causal factor of RGC dysfunction. In addition to elevated IOP, different insults may conjure up to induce RGC death in glaucoma.38, 39 In DBA/2J mice these include excitotoxicity, axonal injury, glial activation, ischemia, and autoimmunity. 40-42 43 Age-dependent, IOP-independent, increase of RGC susceptibility in mice has also been reported in response to optic nerve crush.44 Finally, RGC susceptibility to optic nerve crush in mice depends on the genetic background.45 Altogether, these results indicate that IOP initiates or amplifies pathogenetic processes of RGCs resulting in progressive reduction of electrical responsiveness. Reduction of RGC electrical responsiveness is at least in part reversible by lowering IOP, thus indicating that a stage of IOP-dependent, reversible RGC dysfunction may precede cell death. The present findings support previous reports of PERG improvement after IOP reduction in patients with glaucoma and ocular hypertension. (reviewed in 46)
Evaluation of PERG changes in response to artificial IOP modulation may represent a powerful tool to non-invasively evaluate RGC susceptibility to IOP insult in genetically distinct mouse models of glaucoma.
Acknowledgements
We wish to thank Allergan, Inc. for supporting Maher Saleh.
Financial support: NIH RO3 EY016322, NIH RO1 EY014957, NIH center grant P30-EY14801, unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc.
References
- 1.John SW, Anderson MG, Smith RS. Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J Glaucoma. 1999;8:400–412. [PubMed] [Google Scholar]
- 2.John SW. Mechanistic insights into glaucoma provided by experimental genetics the cogan lecture. Invest Ophthalmol Vis Sci. 2005;46:2649–2661. doi: 10.1167/iovs.05-0205. [DOI] [PubMed] [Google Scholar]
- 3.Lindsey JD, Weinreb RN. Elevated intraocular pressure and transgenic applications in the mouse. J Glaucoma. 2005;14:318–320. doi: 10.1097/01.ijg.0000169411.09258.f6. [DOI] [PubMed] [Google Scholar]
- 4.Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002;42:471–478. doi: 10.1016/s0042-6989(01)00194-8. [DOI] [PubMed] [Google Scholar]
- 5.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 6.Libby RT, Anderson MG, Pang IH, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- 7.Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichstein D, Ren L, Filippopoulos T, Mittag T, Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13–21. doi: 10.1016/j.exer.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 10.Porciatti V, Pizzorusso T, Cenni MC, Maffei L. The visual response of retinal ganglion cells is not altered by optic nerve transection in transgenic mice overexpressing Bcl-2. Proc Natl Acad Sci U S A. 1996;93:14955–14959. doi: 10.1073/pnas.93.25.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porciatti V, Saleh M, Nagaraju M. The pattern electroretinogram as a tool to monitor progressive retinal ganglion cell dysfunction in the DBA/2J mouse model of glaucoma. Invest Ophthalmol. 2007;48:745–751. doi: 10.1167/iovs.06-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007 doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh M, Nagaraju M, Porciatti V. The natural history of retinal ganglion cells and its relationship with IOP in DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48 doi: 10.1167/iovs.07-0582. E-Abstract 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aihara M, Lindsey JD, Weinreb RN. Episcleral venous pressure of mouse eye and effect of body position. Curr Eye Res. 2003;27:355–362. doi: 10.1076/ceyr.27.6.355.18194. [DOI] [PubMed] [Google Scholar]
- 15.Friberg TR, Sanborn G. Optic nerve dysfunction during gravity inversion. Pattern reversal visual evoked potentials. Arch Ophthalmol. 1985;103:1687–1689. doi: 10.1001/archopht.1985.01050110081030. [DOI] [PubMed] [Google Scholar]
- 16.Avila MY, Carre DA, Stone RA, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001;42:1841–1846. [PubMed] [Google Scholar]
- 17.Porciatti V, Libby RT, Lee RK, John SW. Int Conf Eye Research (ICER) Buenos Aires: 2006. Functional changes of retinal ganglion cells in the DBA/2J mouse model of glaucoma. [Google Scholar]
- 18.Nagaraju M, Saleh M, Porciatti V. Postural changes of IOP and pattern ERG in DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48 doi: 10.1167/iovs.07-0582. E-Abstract 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danias J, Kontiola AI, Filippopoulos T, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 20.Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 21.Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 22.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 23.Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Calderone L, Grimes P, Shalev M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res. 1986;42:331–337. doi: 10.1016/0014-4835(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.John SW, Hagaman JR, MacTaggart TE, Peng L, Smithes O. Intraocular pressure in inbred mouse strains. Invest Ophthalmol Vis Sci. 1997;38:249–253. [PubMed] [Google Scholar]
- 27.Aihara M, Lindsey JD, Weinreb RN. Reduction of intraocular pressure in mouse eyes treated with latanoprost. Invest Ophthalmol Vis Sci. 2002;43:146–150. [PubMed] [Google Scholar]
- 28.Nissirios N, Goldblum D, Rohrer K, Mittag T, Danias J. Noninvasive determination of intraocular pressure (IOP) in nonsedated mice of 5 different inbred strains. J Glaucoma. 2007;16:57–61. doi: 10.1097/IJG.0b013e31802b3547. [DOI] [PubMed] [Google Scholar]
- 29.Krieglstein GK, Waller WK, Leydhecker W. The vascular basis of the positional influence of the intraocular pressure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;206:99–106. doi: 10.1007/BF00414618. [DOI] [PubMed] [Google Scholar]
- 30.Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987;103:523–526. doi: 10.1016/s0002-9394(14)74275-8. [DOI] [PubMed] [Google Scholar]
- 31.Fisher RF. Value of tonometry and tonography in the diagnosis of glaucoma. Br J Ophthalmol. 1972;56:200–204. doi: 10.1136/bjo.56.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson DR, Grant WM. The influence of position on intraocular pressure. Invest Ophthalmol. 1973;12:204–212. [PubMed] [Google Scholar]
- 33.Schuman JS, Massicotte EC, Connolly S, Hertzmark E, Mukherji B, Kunen MZ. Increased intraocular pressure and visual field defects in high resistance wind instrument players. Ophthalmology. 2000;107:127–133. doi: 10.1016/s0161-6420(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avila MY, Mitchell CH, Stone RA, Civan MM. Noninvasive assessment of aqueous humor turnover in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44:722–727. doi: 10.1167/iovs.02-0386. [DOI] [PubMed] [Google Scholar]
- 36.Singleton CD, Robertson D, Byrne DW, Joos KM. Effect of posture on blood and intraocular pressures in multiple system atrophy, pure autonomic failure, and baroreflex failure. Circulation. 2003;108:2349–2354. doi: 10.1161/01.CIR.0000097114.11038.26. [DOI] [PubMed] [Google Scholar]
- 37.Savinova OV, Sugiyama F, Martin JE, et al. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42. doi: 10.1016/s0039-6257(05)80042-6. [DOI] [PubMed] [Google Scholar]
- 39.Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- 40.Mo JS, Anderson MG, Gregory M, et al. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J Exp Med. 2003;197:1335–1344. doi: 10.1084/jem.20022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuettauf F, Rejdak R, Walski M, et al. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol (Berl) 2004;107:352–358. doi: 10.1007/s00401-003-0816-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005;280:31240–31248. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]
- 43.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 44.Wang AL, Yuan M, Neufeld AH. Age-related changes in neuronal susceptibility to damage: comparison of the retinal ganglion cells of young and old mice before and after optic nerve crush. Ann N Y Acad Sci. 2007;1097:64–66. doi: 10.1196/annals.1379.027. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Semaan SJ, Schlamp CL, Nickells RW. Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice. BMC Neurosci. 2007;8:19. doi: 10.1186/1471-2202-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology. 2005;112:20–27. doi: 10.1016/j.ophtha.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]