Abstract
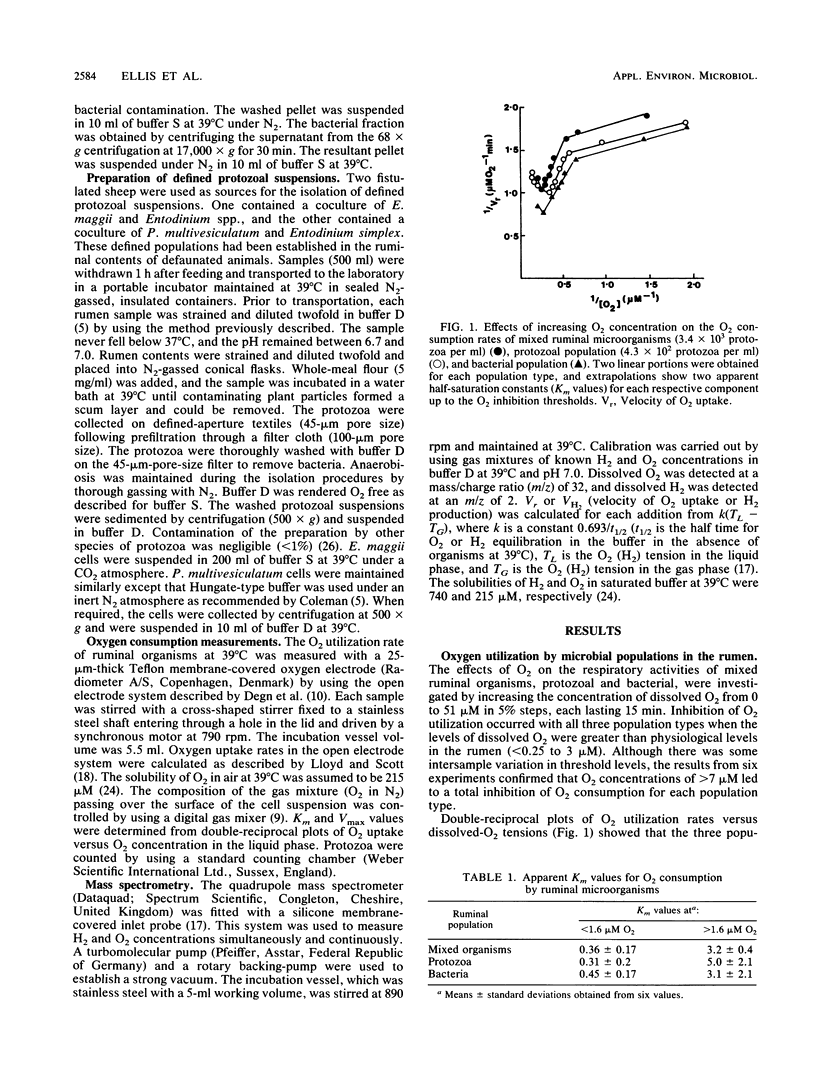
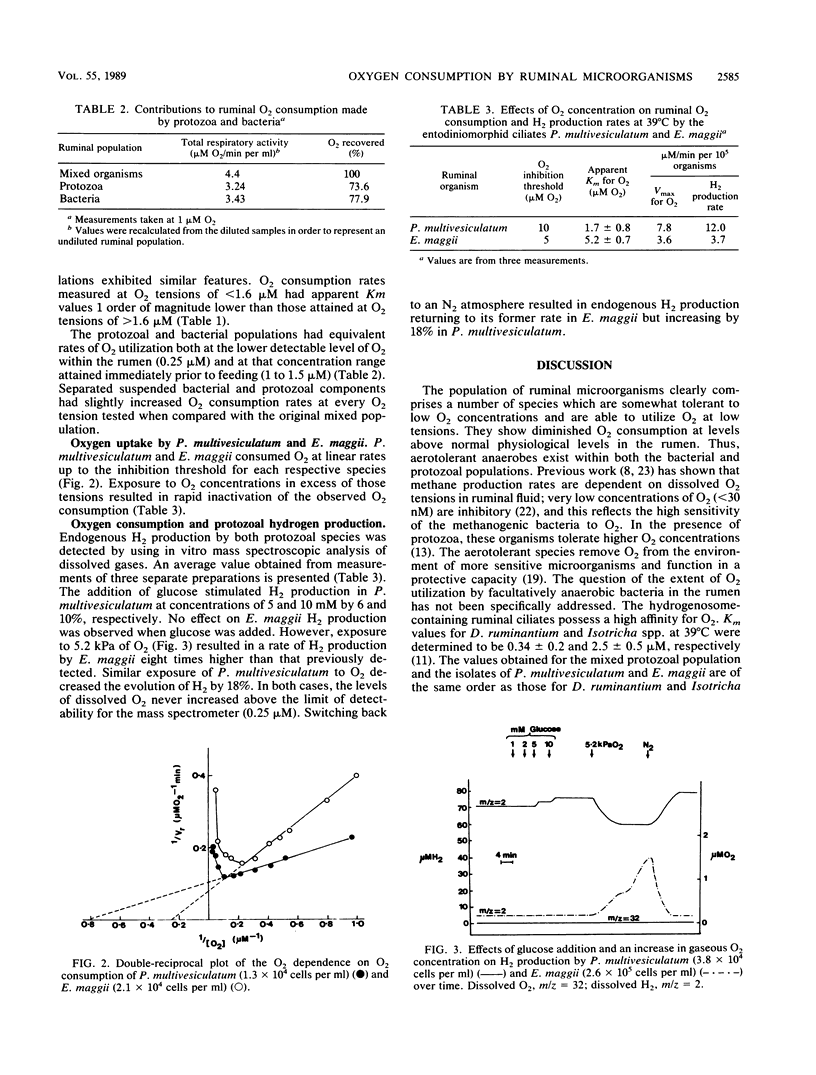
The relative contributions to O2 consumption made by the protozoal and bacterial populations present within the rumen were determined by using an open-type oxygen electrode system. Measurements indicated that two separate microbial populations contributed approximately equally to ruminal O2 consumption over the O2 concentration range experienced in situ (0.25 to 1.0 microM). The populations were observed to consume O2 under liquid-phase O2 concentrations of up to 7 microM, above which point rapid inactivation of O2 utilization was observed. Km values for the mixed population of bacteria and protozoa were 0.36 +/- 0.17 and 3.2 +/- 0.4 microM at concentrations of less than 1.6 and greater than 1.6 microM, respectively. O2 affinity values obtained for both the protozoal and bacterial populations were similar. O2 affinities of the isolated entodiniomorphid ciliates Polyplastron multivesiculatum and Eudiplodinium maggii showed O2 inhibition thresholds of 10 and 5, respectively, and apparent half-saturation constants (Km values) of 1.7 and 5.2 microM O2, respectively. Corresponding Vmax values were 7.8 microM O2 per min per 10(5) organisms for P. multivesiculatum and 3.6 microM O2 per min per 10(5) organisms for E. maggii. Mass spectroscopic analysis detected average rates of H2 production of 12.0 and 3.7 microM H2 per min per 10(5) organisms for P. multivesiculatum and E. maggii, respectively. Trace levels of dissolved O2 (less than 0.25 microM) stimulated the H2 production rate of E. maggii eightfold but inhibited that of P. multivesiculatum by 18%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L., Allison M. J. Rumen metabolism. J Anim Sci. 1983 Jul;57 (Suppl 2):461–477. [PubMed] [Google Scholar]
- Cerkasov J., Cerkasovová A., Kulda J., Vilhelmová D. Respiration of hydrogenosomes of Tritrichomonas foetus. I. ADP-dependent oxidation of malate and pyruvate. J Biol Chem. 1978 Feb 25;253(4):1207–1214. [PubMed] [Google Scholar]
- Czerkawski J. W., Breckenridge G. The effect of oxygen on fermentation of sucrose by rumen micro-organisms in vitro. Br J Nutr. 1969 Mar;23(1):67–80. doi: 10.1079/bjn19690010. [DOI] [PubMed] [Google Scholar]
- Czerkawski J. W. Methane production in ruminants and its significance. World Rev Nutr Diet. 1969;11:240–282. doi: 10.1159/000387580. [DOI] [PubMed] [Google Scholar]
- Degn H., Lundsgaard J. S. Dynamic gas mixing techniques. J Biochem Biophys Methods. 1980 Oct;3(4):233–242. doi: 10.1016/0165-022x(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Degn H., Lundsgaard J. S., Petersen L. C., Ormicki A. Polarographic measurement of steady state kinetics of oxygen uptake by biochemical samples. Methods Biochem Anal. 1980;26:47–77. doi: 10.1002/9780470110461.ch2. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Lloyd D., Williams J., Yarlett N., Williams A. G. Oxygen affinities of the hydrogenosome-containing protozoa Tritrichomonas foetus and Dasytricha ruminantium, and two aerobic protozoa, determined by bacterial bioluminescence. J Gen Microbiol. 1982 May;128(5):1019–1022. doi: 10.1099/00221287-128-5-1019. [DOI] [PubMed] [Google Scholar]
- Müller M., Lindmark D. G. Respiration of hydrogenosomes of Tritrichomonas foetus. II. Effect of CoA on pyruvate oxidation. J Biol Chem. 1978 Feb 25;253(4):1215–1218. [PubMed] [Google Scholar]
- Williams A. G. Rumen holotrich ciliate protozoa. Microbiol Rev. 1986 Mar;50(1):25–49. doi: 10.1128/mr.50.1.25-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. Hydrogenosomes in the rumen protozoon Dasytricha ruminantium Schuberg. Biochem J. 1981 Nov 15;200(2):365–372. doi: 10.1042/bj2000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Lloyd D., Williams A. G. Respiration of the rumen ciliate Dasytricha ruminantium Schuberg. Biochem J. 1982 Aug 15;206(2):259–266. doi: 10.1042/bj2060259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Orpin C. G., Munn E. A., Yarlett N. C., Greenwood C. A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J. 1986 Jun 15;236(3):729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]


