Abstract
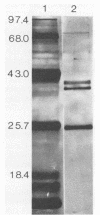
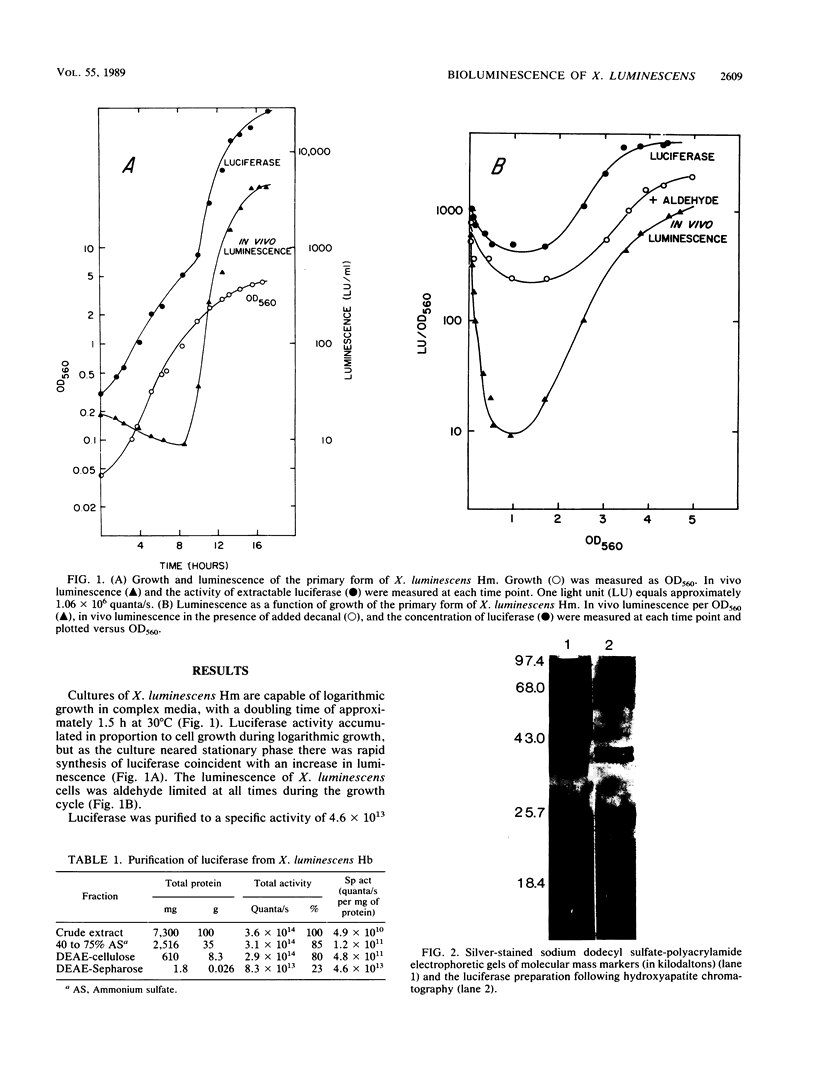
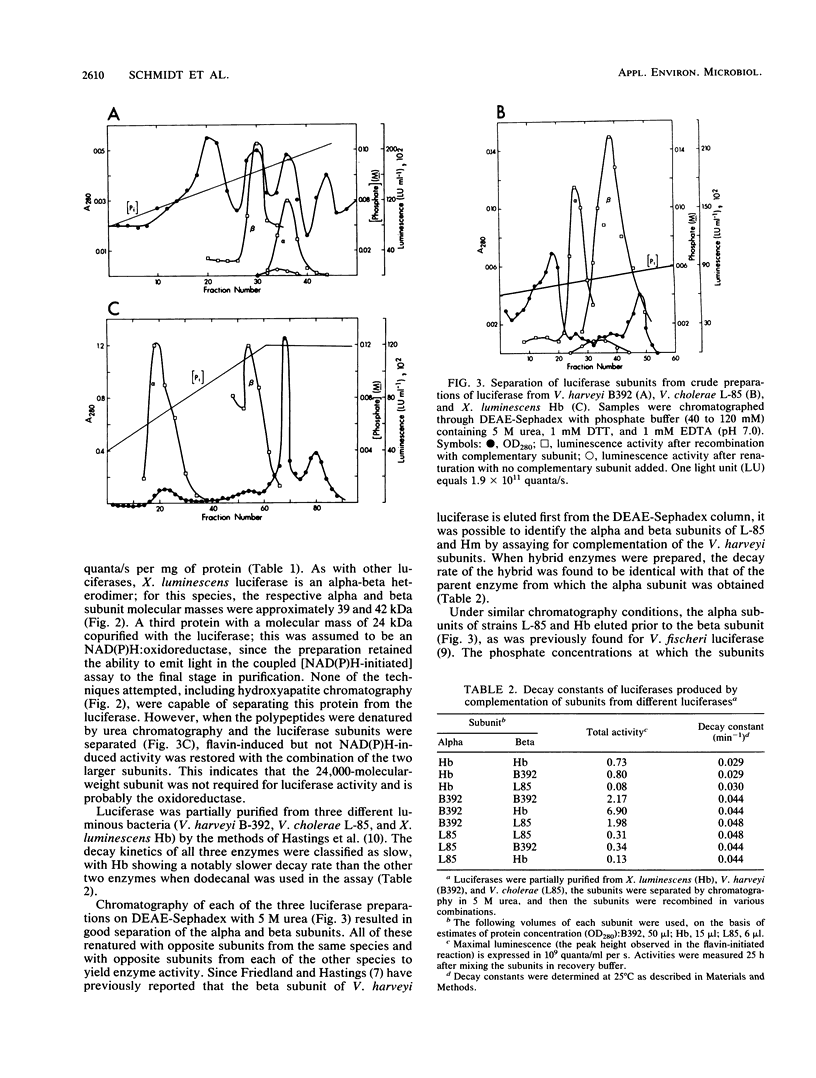
Luminescence of batch cultures of Xenorhabdus luminescens was maximal when cultures approached stationary phase; the onset of in vivo luminescence coincided with a burst of synthesis of bacterial luciferase, the enzyme responsible for luminescence. Expression of luciferase was aldehyde limited at all stages of growth, although more so during the preinduction phase. Luciferase was purified from cultures of X. luminescens Hm to a specific activity of 4.6 x 10(13) guanta/s per mg of protein and found to be similar to other bacterial luciferases. The Xenorhabdus luciferase consisted of two subunits with approximate molecular masses of 39 and 42 kilodaltons. A third protein with a molecular mass of 24 kilodaltons copurified with luciferase, and in its presence, either NADH or NADPH was effective in stimulating luminescence, indicating that this protein is an NAD(P)H oxidoreductase. Luciferases from two other luminous bacteria, Vibrio harveyii (B392) and Vibrio cholerae (L85), were partially purified, and their subunits were separated in 5 M urea and tested for complementation with the subunits prepared from X. luminescens Hb. Positive complementation was seen with luciferase subunits among all three species. The slow decay kinetics of the Xenorhabdus luciferase were attributed to the alpha subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. O., Ziegler M. M., Powers D. A. Covalent structure of subunits of bacterial luciferase: NH2-terminal sequence demonstrates subunit homology. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4887–4889. doi: 10.1073/pnas.76.10.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Mileham A. J., Simon M. I., Nealson K. H., Rausch S. K., Bonam D., Baldwin T. O. Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the alpha subunit of bacterial luciferase. J Biol Chem. 1985 May 25;260(10):6139–6146. [PubMed] [Google Scholar]
- Colepicolo P., Cho K. W., Poinar G. O., Hastings J. W. Growth and luminescence of the bacterium Xenorhabdus luminescens from a human wound. Appl Environ Microbiol. 1989 Oct;55(10):2601–2606. doi: 10.1128/aem.55.10.2601-2606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J., Hastings J. W. Nonidentical subunits of bacterial luciferase: their isolation and recombination to form active enzyme. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2336–2342. doi: 10.1073/pnas.58.6.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- Hastings J. W., Potrikus C. J., Gupta S. C., Kurfürst M., Makemson J. C. Biochemistry and physiology of bioluminescent bacteria. Adv Microb Physiol. 1985;26:235–291. doi: 10.1016/s0065-2911(08)60398-7. [DOI] [PubMed] [Google Scholar]
- Jablonski E., DeLuca M. Purification and properties of the NADH and NADPH specific FMN oxidoreductases from Beneckea harveyi. Biochemistry. 1977 Jun 28;16(13):2932–2936. doi: 10.1021/bi00632a020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michaliszyn G. A., Wing S. S., Meighen E. A. Purification and properties of a NAD(P)H:flavin oxidoreductase from the luminous bacterium, Beneckea harveyi. J Biol Chem. 1977 Nov 10;252(21):7495–7499. [PubMed] [Google Scholar]
- Mosher D. F., Schad P. E., Vann J. M. Cross-linking of collagen and fibronectin by factor XIIIa. Localization of participating glutaminyl residues to a tryptic fragment of fibronectin. J Biol Chem. 1980 Feb 10;255(3):1181–1188. [PubMed] [Google Scholar]
- Richardson W. H., Schmidt T. M., Nealson K. H. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl Environ Microbiol. 1988 Jun;54(6):1602–1605. doi: 10.1128/aem.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Hastings J. W. Formation of hybrid luciferases from subunits of different species of Photobacterium. Biochemistry. 1980 Oct 28;19(22):4989–4993. doi: 10.1021/bi00563a009. [DOI] [PubMed] [Google Scholar]
- Schmidt T. M., Bleakley B., Nealson K. H. Characterization of an Extracellular Protease from the Insect Pathogen Xenorhabdus luminescens. Appl Environ Microbiol. 1988 Nov;54(11):2793–2797. doi: 10.1128/aem.54.11.2793-2797.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T. S. Separation of bacterial luciferase from oxidoreductases by affinity chromatography. Anal Biochem. 1985 Nov 15;151(1):137–141. doi: 10.1016/0003-2697(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Tu S. C., Hastings J. W. Physical interaction and activity coupling between two enzymes induced by immobilization of one. Proc Natl Acad Sci U S A. 1980 Jan;77(1):249–252. doi: 10.1073/pnas.77.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Hastings J. W. Specificities and properties of three reduced pyridine nucleotide-flavin mononucleotide reductases coupling to bacterial luciferase. Mol Cell Biochem. 1982 May 14;44(3):181–187. doi: 10.1007/BF00238506. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]