Abstract
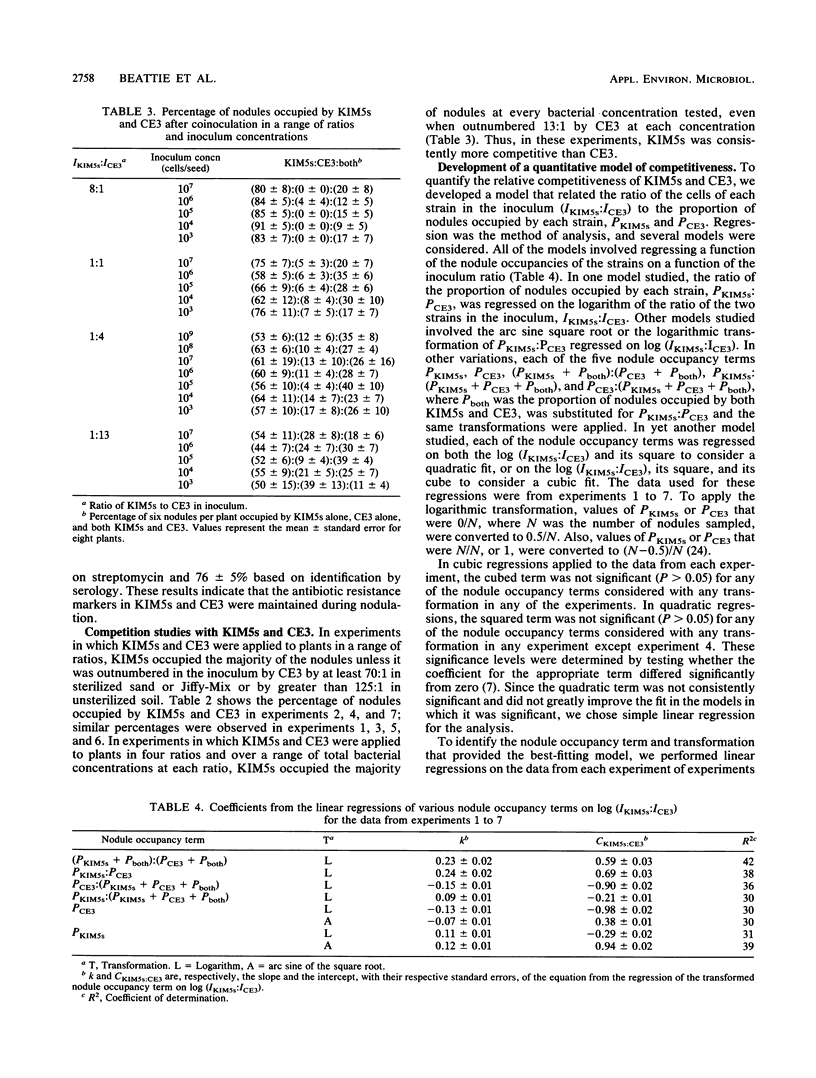
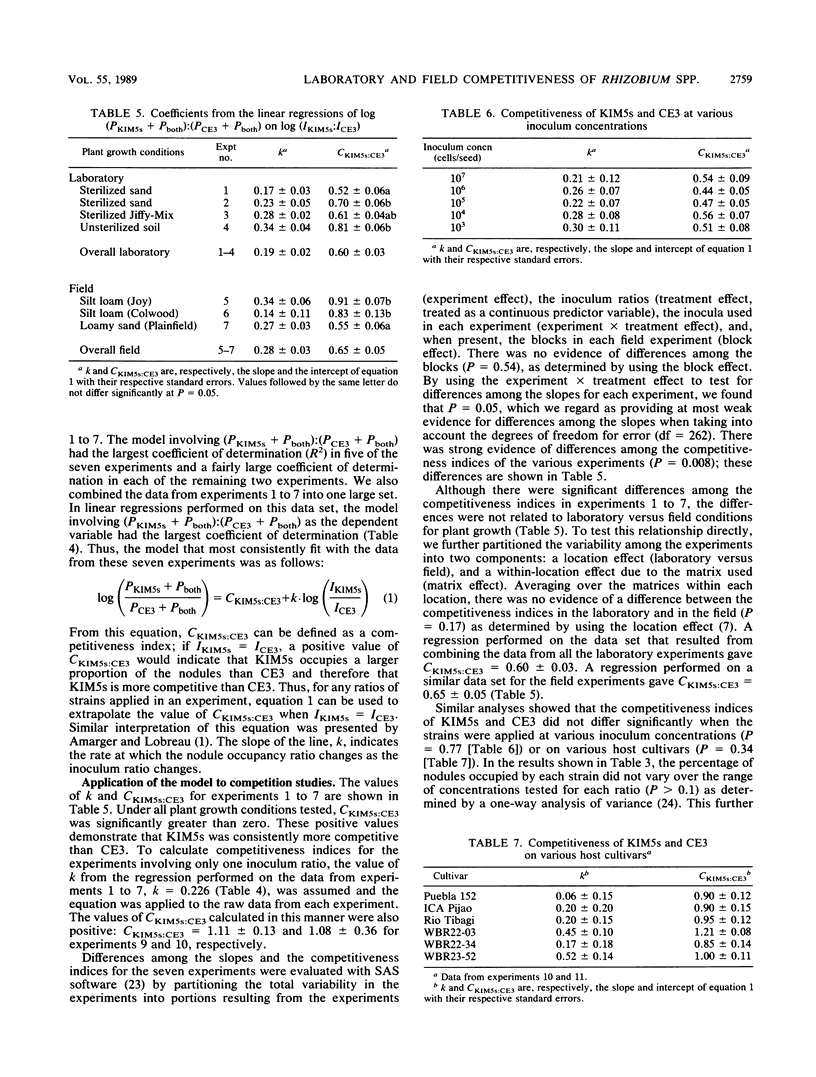
Rhizobium leguminosarum bv. phaseoli KIM5s outcompeted strain CE3 in bean (Phaseolus vulgaris L.) root nodulation when plants were grown at any of three field sites, each with a different soil type and indigenous population, or in the laboratory in a sterilized sand, a sterilized peat-vermiculite mixture, or a nonsterile field soil. A mathematical model describing nodulation competitiveness was empirically derived to evaluate the relative competitiveness of the two strains under these conditions. This model relates the proportional representation of the two strains in the inoculum to the proportional representation of nodules occupied by each strain or both strains and provides a measure of competitiveness, which is referred to as the competitiveness index. Statistical comparisons of competitiveness indices showed that the relative competitiveness of KIM5s and CE3 remained constant when the two strains were applied in a constant ratio over a range of inoculum concentrations, from 10(3) to 10(7) cells per seed, and when they were applied in various ratios to six P. vulgaris cultivars. Furthermore, the relative competitiveness of KIM5s and CE3 in the laboratory did not differ significantly from their relative competitiveness at the three field sites studied. Thus, a study of the basis for nodulation competitiveness of KIM5s and CE3 in the laboratory has the potential to provide an understanding of competitiveness both in the laboratory and in the field.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P., Bergman K. Competitive advantage provided by bacterial motility in the formation of nodules by Rhizobium meliloti. J Bacteriol. 1981 Nov;148(2):728–p. doi: 10.1128/jb.148.2.728-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo R. S., Maya-Flores J., Barnes-McConnell D., Yokoyama C., Dazzo F. B., Bliss F. A. Semienclosed Tube Cultures of Bean Plants (Phaseolus vulgaris L.) for Enumeration of Rhizobium phaseoli by the Most-Probable-Number Technique. Appl Environ Microbiol. 1986 Oct;52(4):954–956. doi: 10.1128/aem.52.4.954-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Barnet Y. M., Vincent J. M. Effect of a bacteriophage on colonisation and nodulation of clover roots by paired strains of Rhizobium trifolii. Can J Microbiol. 1979 Sep;25(9):974–978. doi: 10.1139/m79-149. [DOI] [PubMed] [Google Scholar]
- Handelsman J., Brill W. J. Erwinia herbicola isolates from alfalfa plants may play a role in nodulation of alfalfa by Rhizobium meliloti. Appl Environ Microbiol. 1985 Apr;49(4):818–821. doi: 10.1128/aem.49.4.818-821.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman J., Ugalde R. A., Brill W. J. Rhizobium meliloti competitiveness and the alfalfa agglutinin. J Bacteriol. 1984 Mar;157(3):703–707. doi: 10.1128/jb.157.3.703-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Mutant Strains of Rhizobium japonicum with Increased Ability to Fix Nitrogen for Soybean. Science. 1978 Aug 4;201(4354):448–450. doi: 10.1126/science.201.4354.448. [DOI] [PubMed] [Google Scholar]
- Materon L. A., Hagedorn C. Competitiveness of Rhizobium trifolii Strains Associated with Red Clover (Trifolium pratense L.) in Mississippi Soils. Appl Environ Microbiol. 1982 Nov;44(5):1096–1101. doi: 10.1128/aem.44.5.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L. Variations in Ability of Rhizobium japonicum Strains To Nodulate Soybeans and Maintain Fixation in the Presence of Nitrate. Appl Environ Microbiol. 1982 Sep;44(3):647–652. doi: 10.1128/aem.44.3.647-652.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J., Higgins P., O'gara F. Studies on the Inoculation and Competitiveness of a Rhizobium leguminosarum Strain in Soils Containing Indigenous Rhizobia. Appl Environ Microbiol. 1985 Apr;49(4):899–903. doi: 10.1128/aem.49.4.899-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf R. C., Jr, Dimond R. L. Functional heterogeneity of monoclonal antibodies obtained using different screening assays. Anal Biochem. 1983 Nov;135(1):221–229. doi: 10.1016/0003-2697(83)90754-6. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Barta T. M. Trifolitoxin Production and Nodulation Are Necessary for the Expression of Superior Nodulation Competitiveness by Rhizobium leguminosarum bv. trifolii Strain T24 on Clover. Plant Physiol. 1987 Oct;85(2):335–342. doi: 10.1104/pp.85.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde R. A., Handelsman J., Brill W. J. Role of galactosyltransferase activity in phage sensitivity and nodulation competitiveness of Rhizobium meliloti. J Bacteriol. 1986 Apr;166(1):148–154. doi: 10.1128/jb.166.1.148-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadisirisuk P., Danso S. K., Hardarson G., Bowen G. D. Influence of Bradyrhizobium japonicum Location and Movement on Nodulation and Nitrogen Fixation in Soybeans. Appl Environ Microbiol. 1989 Jul;55(7):1711–1716. doi: 10.1128/aem.55.7.1711-1716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


