Abstract
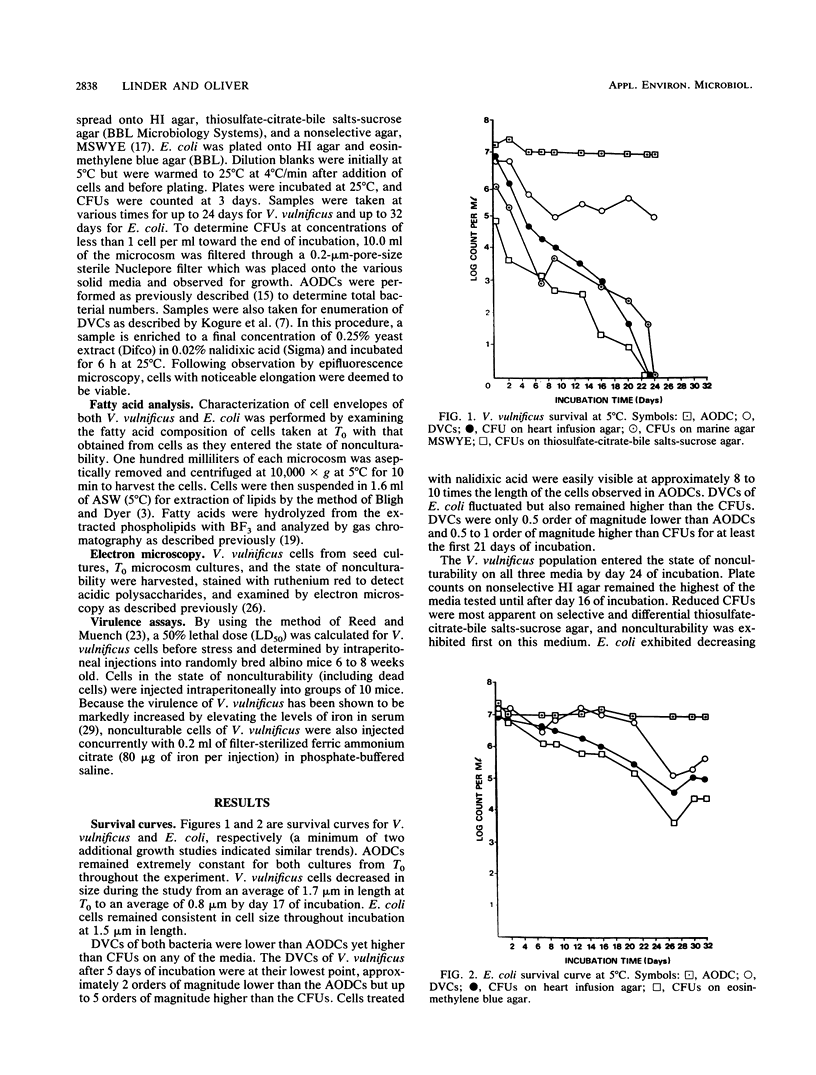
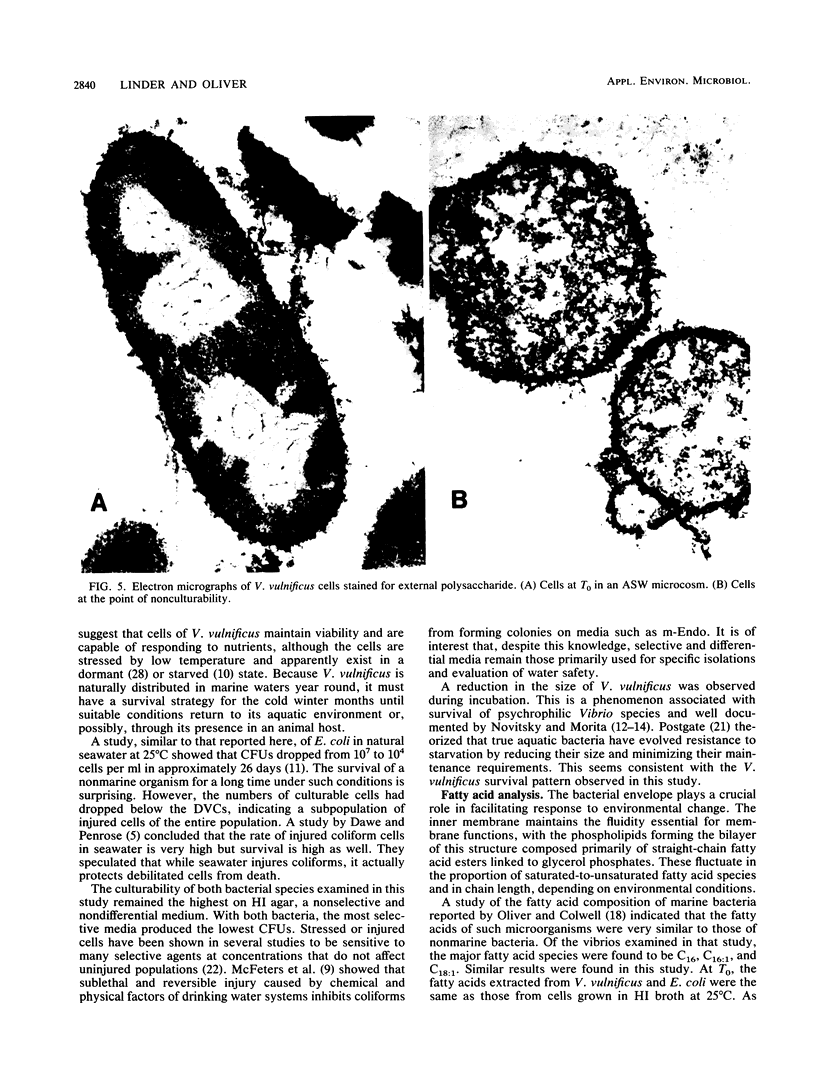
The nonculturable state of Vibrio vulnificus and, for comparison, that of Escherichia coli were studied in artificial-seawater microcosms at 5 degrees C. Total cell counts were monitored by acridine orange epifluorescence, metabolic activity by direct viable counts, and culturability by plate counts on selective and nonselective media. Whereas total counts remained constant, plate counts of V. vulnificus suggested nonculturability by day 24. In contrast, direct viable counts indicated significant cell viability throughout 32 days of incubation. As an indication of the metabolic changes that occurred as cells entered the state of nonrecoverability, membrane fatty acid analyses were performed. At the point of nonculturability of V. vulnificus, the major fatty acid species (C16 and C16:1) had decreased 57% from the T0 level, concomitant with the appearance of several short-chain acids. Although the bacteria were still recoverable, a similar trend was observed with E. coli. Electron microscopy of nonculturable V. vulnificus showed that the cells were rounded and reduced in size and contained fewer ribosomes. Mouse infectivity studies conducted with these cells suggested loss of virulence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Okada K., Miake S. Evidence for the presence of a capsule in Vibrio vulnificus. J Gen Microbiol. 1984 Oct;130(10):2741–2743. doi: 10.1099/00221287-130-10-2741. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Dawe L. L., Penrose W. R. "Bactericidal" property of seawater: death or debilitation? Appl Environ Microbiol. 1978 May;35(5):829–833. doi: 10.1128/aem.35.5.829-833.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D., Testa J. Protection of mice against Vibrio vulnificus disease by vaccination with surface antigen preparations and anti-surface antigen antisera. Infect Immun. 1984 Sep;45(3):537–543. doi: 10.1128/iai.45.3.537-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Kippin J. S., LeChevallier M. W. Injured coliforms in drinking water. Appl Environ Microbiol. 1986 Jan;51(1):1–5. doi: 10.1128/aem.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro P. M., Gauthier M. J., Laumond F. M. Changes in Escherichia coli cells starved in seawater or grown in seawater-wastewater mixtures. Appl Environ Microbiol. 1987 Jul;53(7):1476–1481. doi: 10.1128/aem.53.7.1476-1481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Colwell R. R. Extractable lipids of gram-negative marine bacteria: phospholipid composition. J Bacteriol. 1973 Jun;114(3):897–908. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Stringer W. F. Lipid Composition of a Psychrophilic Marine Vibrio sp. During Starvation-Induced Morphogenesis. Appl Environ Microbiol. 1984 Mar;47(3):461–466. doi: 10.1128/aem.47.3.461-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole M. D., Oliver J. D. Experimental pathogenicity and mortality in ligated ileal loop studies of the newly reported halophilic lactose-positive Vibrio sp. Infect Immun. 1978 Apr;20(1):126–129. doi: 10.1128/iai.20.1.126-129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylski K. S., Witter L. D. Injury and recovery of Escherichia coli after sublethal acidification. Appl Environ Microbiol. 1979 Feb;37(2):261–265. doi: 10.1128/aem.37.2.261-265.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Simpson L. M., White V. K., Zane S. F., Oliver J. D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987 Jan;55(1):269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., McFeters G. A. Survival and virulence of copper- and chlorine-stressed Yersinia enterocolitica in experimentally infected mice. Appl Environ Microbiol. 1987 Aug;53(8):1768–1774. doi: 10.1128/aem.53.8.1768-1774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. C., Simpson L. M., Oliver J. D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981 Nov;34(2):503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Ogawa M., Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985 Feb;47(2):446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



