Abstract
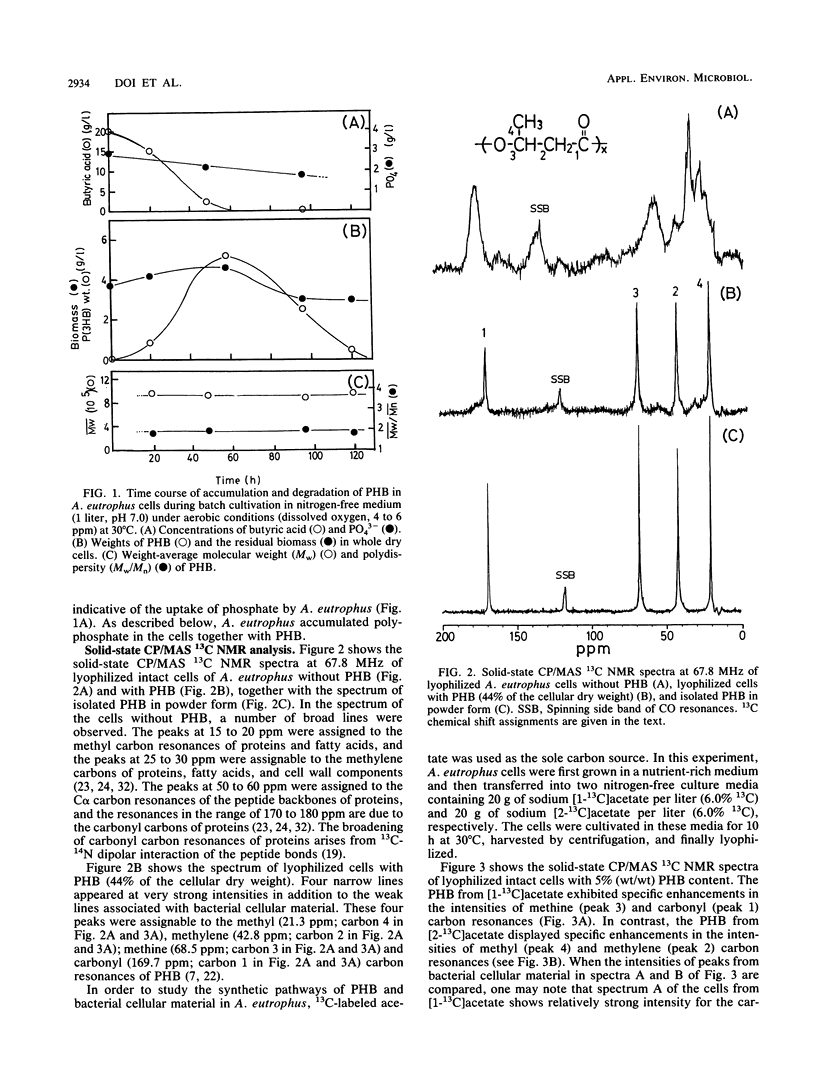
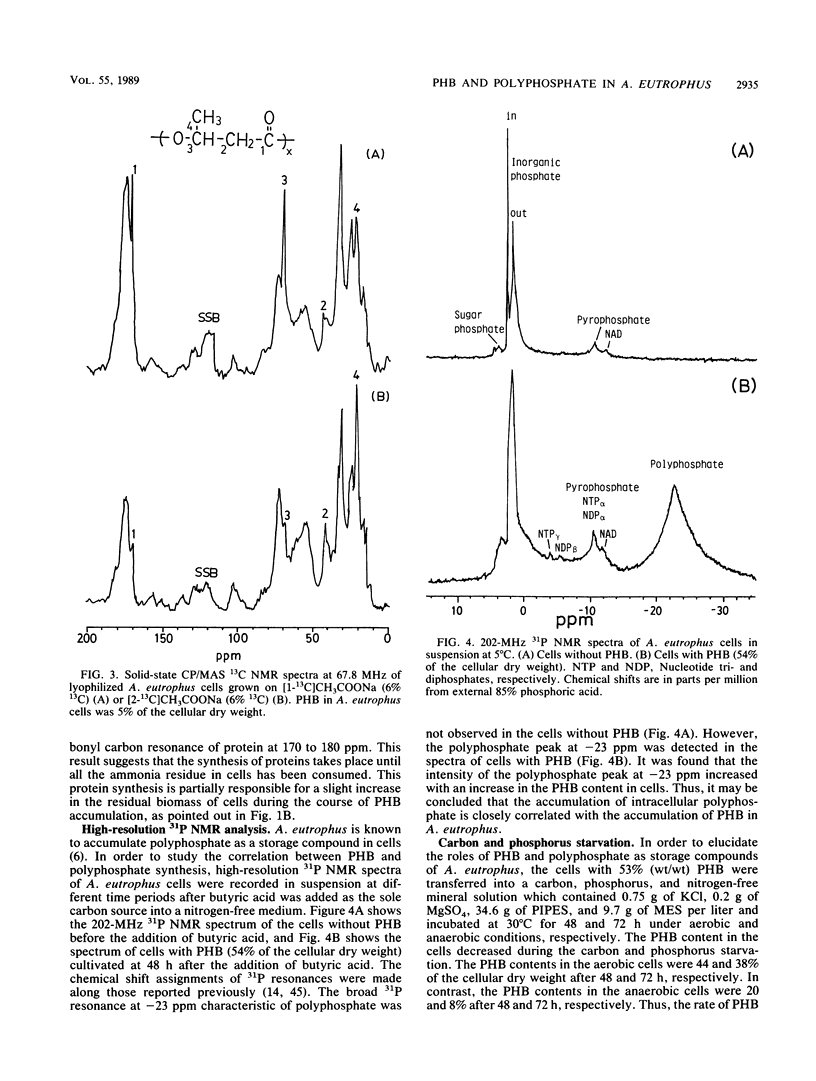
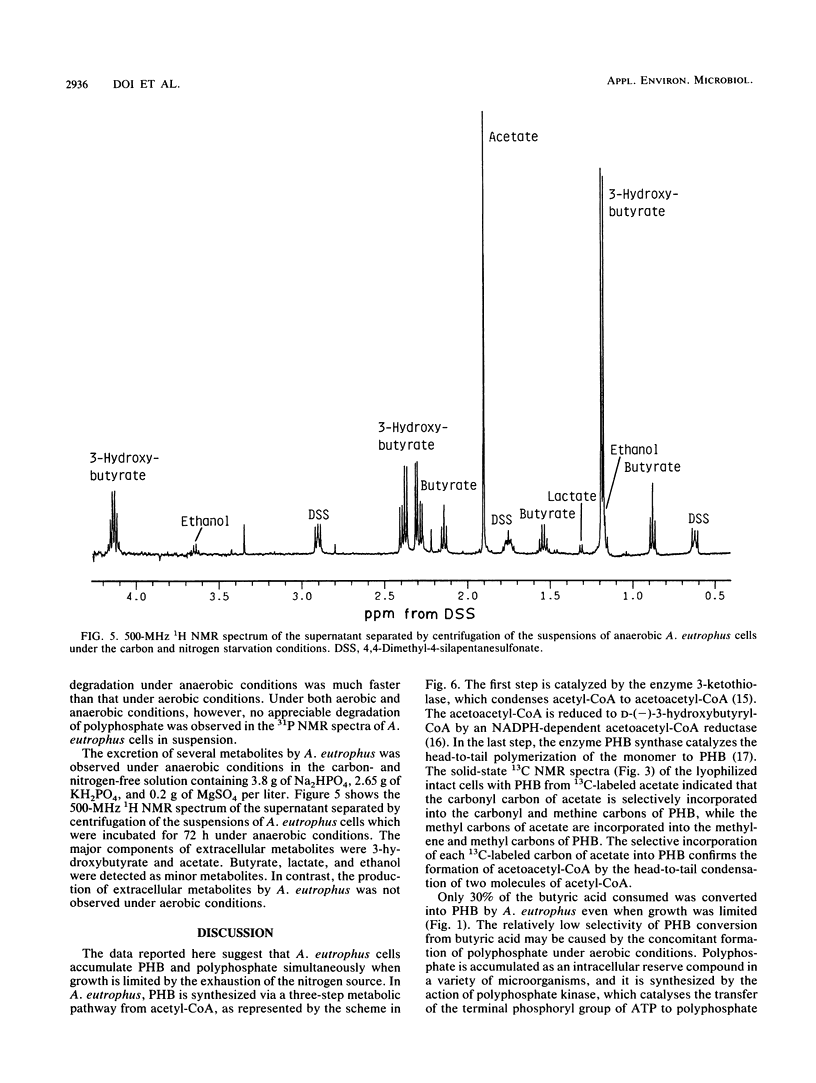
The metabolic pathways of poly(3-hydroxybutyrate) (PHB) and polyphosphate in the microorganism Alcaligenes eutrophus H16 were studied by 1H, 13C, and 31P nuclear magnetic resonance (NMR) spectroscopy and by conventional analytical techniques. A. eutrophus cells accumulated two storage polymers of PHB and polyphosphate in the presence of carbon and phosphate sources under aerobic conditions after exhaustion of nitrogen sources. The solid-state cross-polarization/magic-angle spinning 13C NMR spectroscopy was used to study the biosynthetic pathways of PHB and other cellular biomass components from 13C-labeled acetate. The solid-state 13C NMR analysis of lyophilized intact cells grown on [1-13C]acetate indicated that the carbonyl carbon of acetate was selectively incorporated both into the carbonyl and methine carbons of PHB and into the carbonyl carbons of proteins. The 31P NMR analysis of A. eutrophus cells in suspension showed that the synthesis of intracellular polyphosphate was closely related to the synthesis of PHB. The roles of PHB and polyphosphate in the cells were studied under conditions of carbon, phosphorus, and nitrogen source starvation. Under both aerobic and anaerobic conditions PHB was degraded, whereas little polyphosphate was degraded. The rate of PHB degradation under anaerobic conditions was faster than that under aerobic conditions. Under anaerobic conditions, acetate and 3-hydroxybutyrate were produced as the major extracellular metabolites. The implications of this observation are discussed in connection with the regulation of PHB and polyphosphate metabolism in A. eutrophus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Knee E. J., Jr, Fuller R. C., Gross R. A., Lenz R. W. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly (beta-hydroxyalkanoates): potential sources for biodegradable polyesters. Int J Biol Macromol. 1989 Feb;11(1):49–55. doi: 10.1016/0141-8130(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Davis J. T., Chen H. H., Moore R., Nishitani Y., Masamune S., Sinskey A. J., Walsh C. T. Biosynthetic thiolase from Zoogloea ramigera. II. Inactivation with haloacetyl CoA analogs. J Biol Chem. 1987 Jan 5;262(1):90–96. [PubMed] [Google Scholar]
- Davis J. T., Moore R. N., Imperiali B., Pratt A. J., Kobayashi K., Masamune S., Sinskey A. J., Walsh C. T., Fukui T., Tomita K. Biosynthetic thiolase from zoogloea ramigera. I. Preliminary characterization and analysis of proton transfer reaction. J Biol Chem. 1987 Jan 5;262(1):82–89. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Ultrastructural study of poly- -hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973 Jun;114(3):1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D., Lundgren D. G., Okamura K., Marchessault R. H. Morphology of poly-beta-hydroxybutyrate granules. J Mol Biol. 1968 Aug 14;35(3):489–502. doi: 10.1016/s0022-2836(68)80009-9. [DOI] [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Ito M., Saito T., Tomita K. Purification and characterization of NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Biochim Biophys Acta. 1987 Feb 23;917(3):365–371. [PubMed] [Google Scholar]
- Fukui T., Yoshimoto A., Matsumoto M., Hosokawa S., Saito T. Enzymatic synthesis of poly-beta-hydroxybutyrate in Zoogloea ramigera. Arch Microbiol. 1976 Nov 2;110(23):149–156. doi: 10.1007/BF00690222. [DOI] [PubMed] [Google Scholar]
- Holland S. J., Jolly A. M., Yasin M., Tighe B. J. Polymers for biodegradable medical devices. II. Hydroxybutyrate-hydroxyvalerate copolymers: hydrolytic degradation studies. Biomaterials. 1987 Jul;8(4):289–295. doi: 10.1016/0142-9612(87)90117-7. [DOI] [PubMed] [Google Scholar]
- Jacob G. S., Garbow J. R., Schaefer J. Direct measurement of poly(beta-hydroxybutyrate) in a pseudomonad by solid-state 13C NMR. J Biol Chem. 1986 Dec 25;261(36):16785–16787. [PubMed] [Google Scholar]
- Jacob G. S., Garbow J. R., Schaefer J., Kishore G. M. Solid-state NMR studies of regulation of N-(phosphonomethyl)glycine and glycine metabolism in Pseudomonas sp. strain PG2982. J Biol Chem. 1987 Feb 5;262(4):1552–1557. [PubMed] [Google Scholar]
- Jacob G. S., Schaefer J., Garbow J. R., Stejskal E. O. Solid-state NMR studies of Klebsiella pneumoniae grown under nitrogen-fixing conditions. J Biol Chem. 1987 Jan 5;262(1):254–259. [PubMed] [Google Scholar]
- KALTWASSER H. [The role of polyphosphate in phosphorus metabolism of an oxyhydrogen gas bacterium (Hydrogenomonas strain 20)]. Arch Mikrobiol. 1962;41:282–306. [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Doudoroff M. Poly-beta-hydroxybutyrate depolymerases of Pseudomonas lemoignei. Proc Natl Acad Sci U S A. 1966 Sep;56(3):960–965. doi: 10.1073/pnas.56.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. DEPOLYMERIZATION OF POLY-BETA-HYDROXYBUTYRATE BY INTRACELLULAR ENZYME SYSTEM. J Bacteriol. 1964 Jul;88:60–71. doi: 10.1128/jb.88.1.60-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. D., Williams D. F. On the biodegradation of poly-beta-hydroxybutyrate (PHB) homopolymer and poly-beta-hydroxybutyrate-hydroxyvalerate copolymers. Biomaterials. 1987 Mar;8(2):129–137. doi: 10.1016/0142-9612(87)90102-5. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Saito T., Fukui T., Shirakura Y., Tomita K. Purification and properties of extracellular poly(3-hydroxybutyrate) depolymerases from Pseudomonas lemoignei. Biochim Biophys Acta. 1985 Jan 21;827(1):63–72. doi: 10.1016/0167-4838(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Saito T., Tomita K. Purification and properties of beta-ketothiolase from Zoogloea ramigera. Arch Microbiol. 1978 Jan 23;116(1):21–27. doi: 10.1007/BF00408729. [DOI] [PubMed] [Google Scholar]
- Oeding V., Schlegel H. G. Beta-ketothiolase from Hydrogenomonas eutropha H16 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J. 1973 May;134(1):239–248. doi: 10.1042/bj1340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples O. P., Masamune S., Walsh C. T., Sinskey A. J. Biosynthetic thiolase from Zoogloea ramigera. III. Isolation and characterization of the structural gene. J Biol Chem. 1987 Jan 5;262(1):97–102. [PubMed] [Google Scholar]
- Ploux O., Masamune S., Walsh C. T. The NADPH-linked acetoacetyl-CoA reductase from Zoogloea ramigera. Characterization and mechanistic studies of the cloned enzyme over-produced in Escherichia coli. Eur J Biochem. 1988 May 16;174(1):177–182. doi: 10.1111/j.1432-1033.1988.tb14079.x. [DOI] [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Fukui T., Ikeda F., Tanaka Y., Tomita K. An NADP-linked acetoacetyl CoA reductase from Zoogloea ramigera. Arch Microbiol. 1977 Sep 28;114(3):211–217. doi: 10.1007/BF00446864. [DOI] [PubMed] [Google Scholar]
- Saito T., Suzuki K., Yamamoto J., Fukui T., Miwa K., Tomita K., Nakanishi S., Odani S., Suzuki J., Ishikawa K. Cloning, nucleotide sequence, and expression in Escherichia coli of the gene for poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. J Bacteriol. 1989 Jan;171(1):184–189. doi: 10.1128/jb.171.1.184-189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakura Y., Fukui T., Saito T., Okamoto Y., Narikawa T., Koide K., Tomita K., Takemasa T., Masamune S. Degradation of poly(3-hydroxybutyrate) by poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis T1. Biochim Biophys Acta. 1986 Jan 15;880(1):46–53. doi: 10.1016/0304-4165(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Saito T., Fukui T., Tanio T., Tomita K. Purification and properties of D(-)-3-hydroxybutyrate-dimer hydrolase from Zoogloea ramigera I-16-M. Eur J Biochem. 1981 Aug;118(1):177–182. doi: 10.1111/j.1432-1033.1981.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Tanio T., Fukui T., Shirakura Y., Saito T., Tomita K., Kaiho T., Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982 May;124(1):71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]


