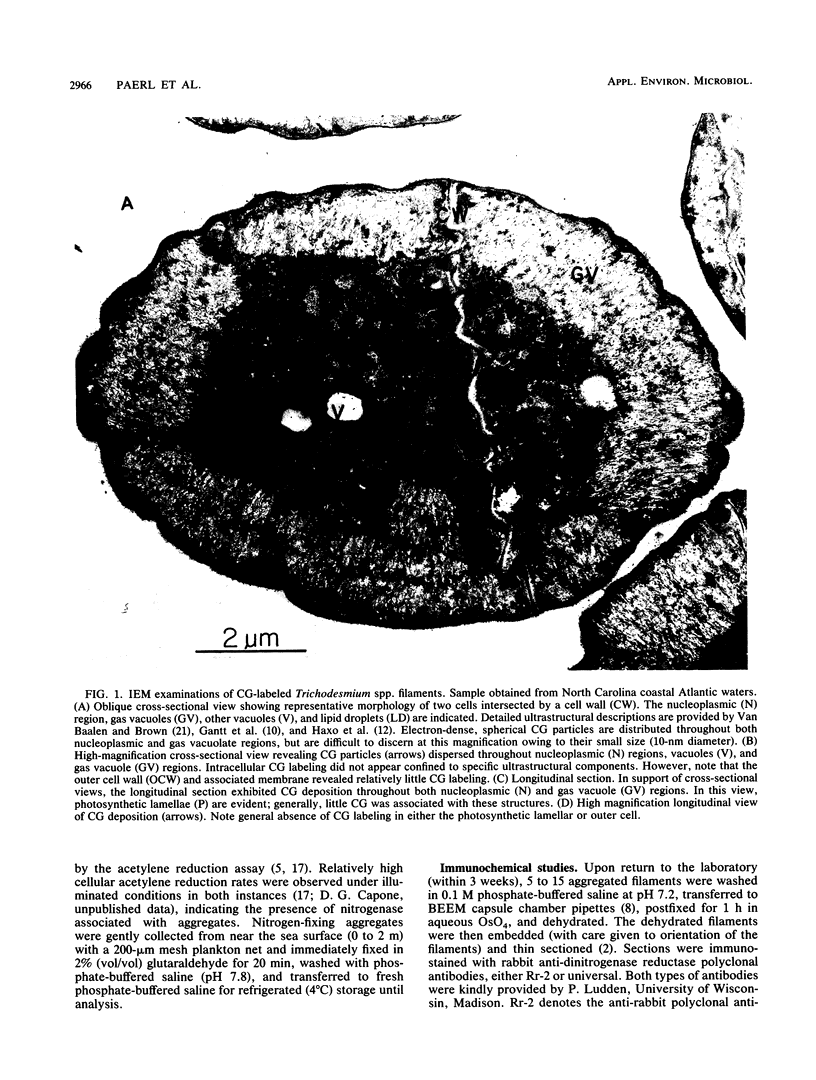
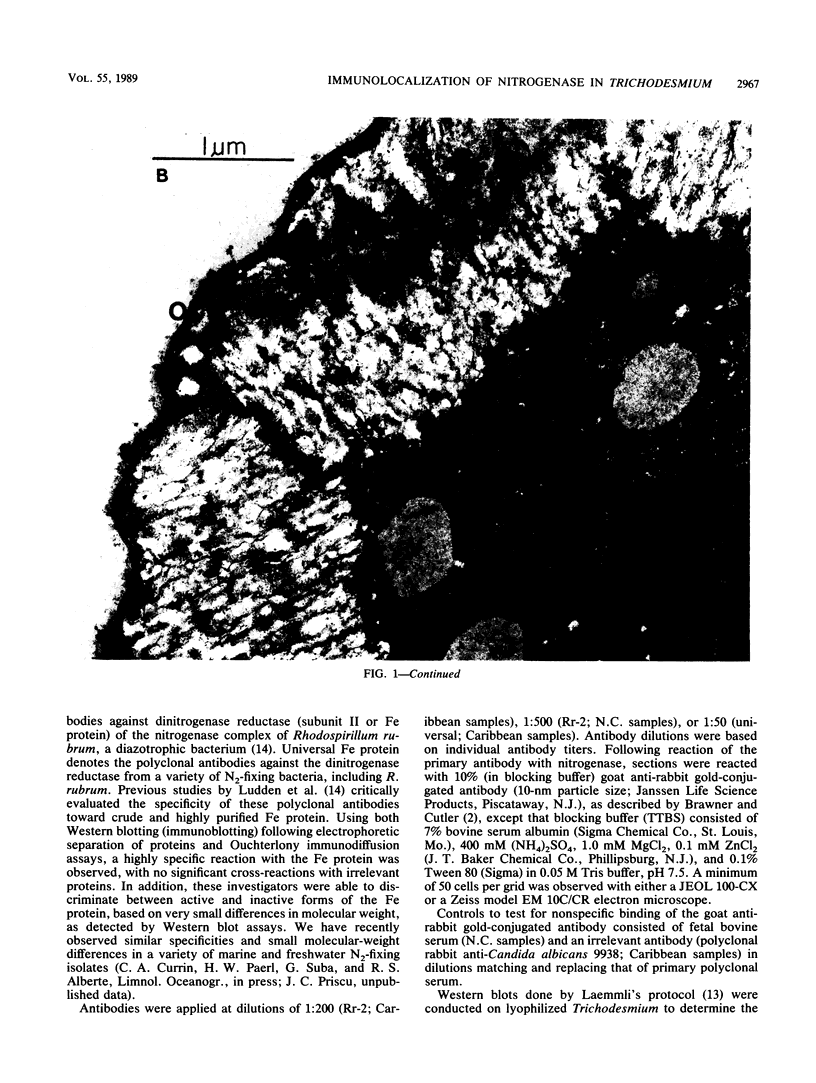
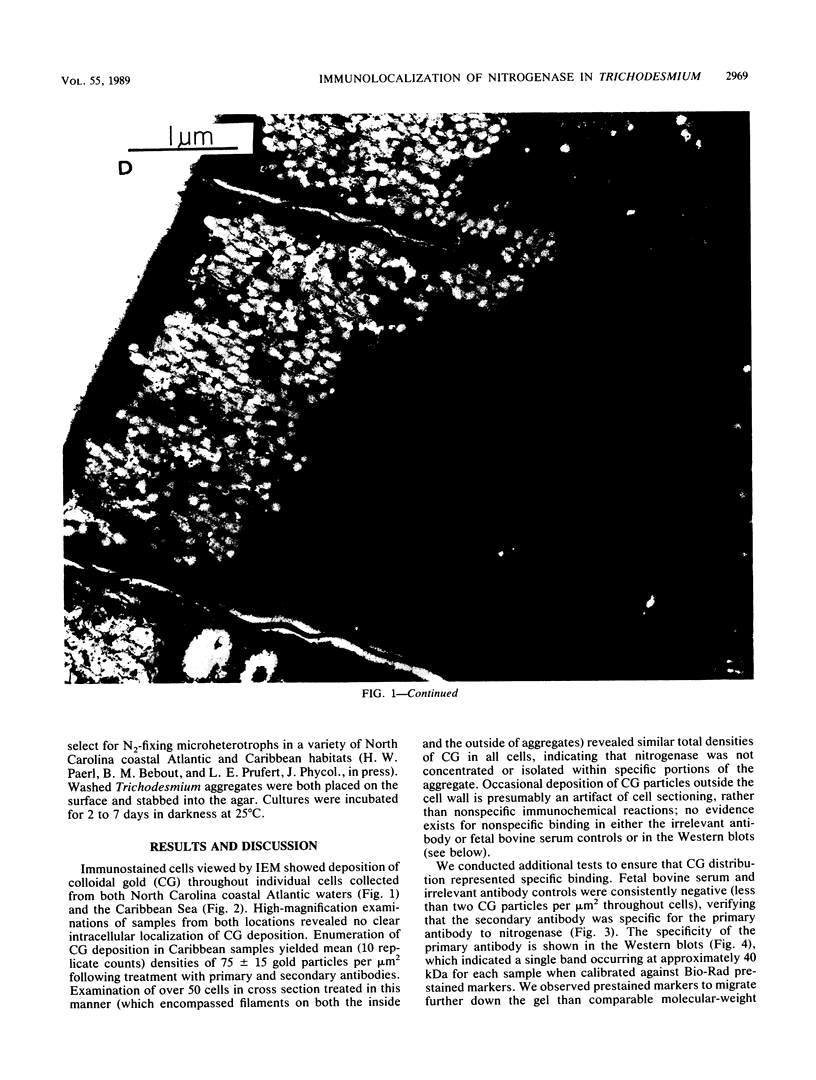
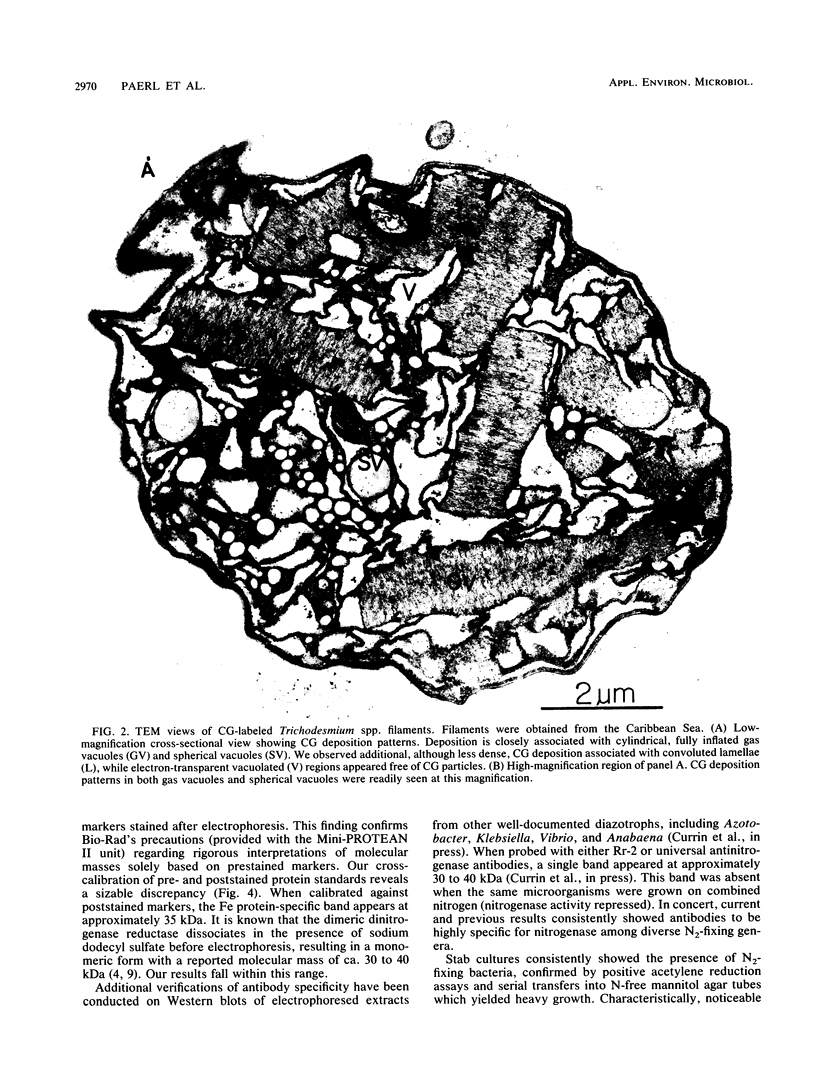
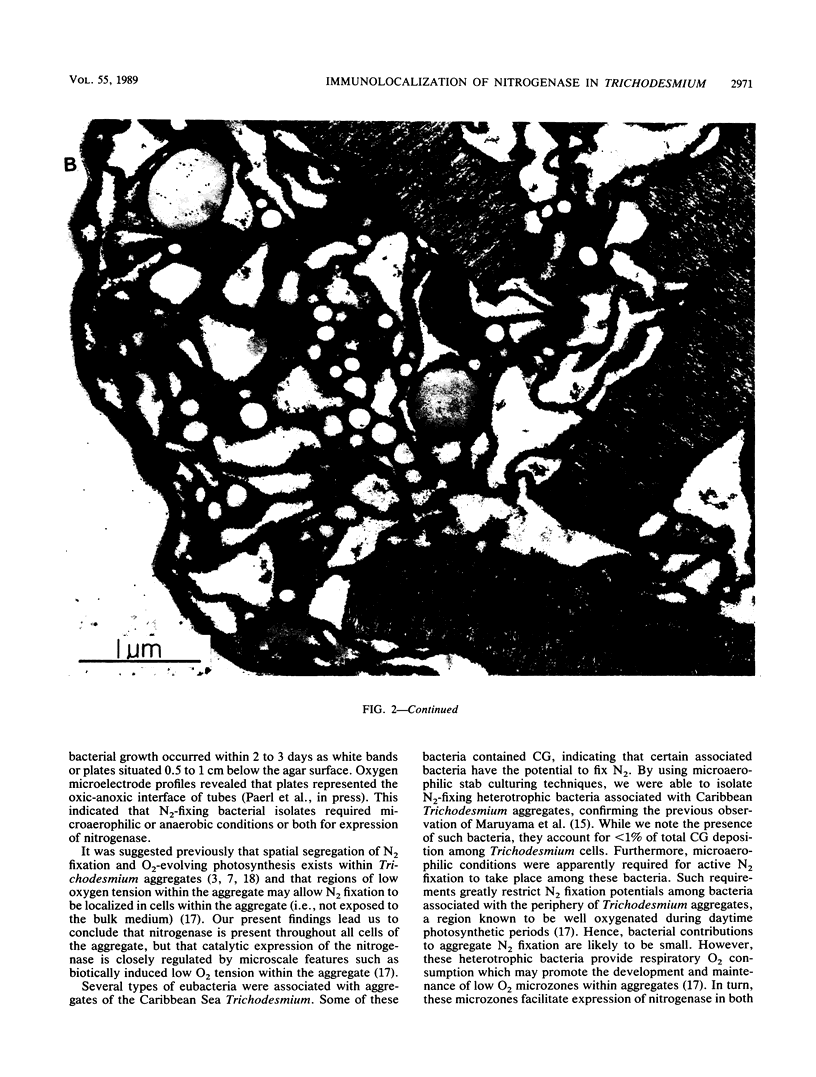
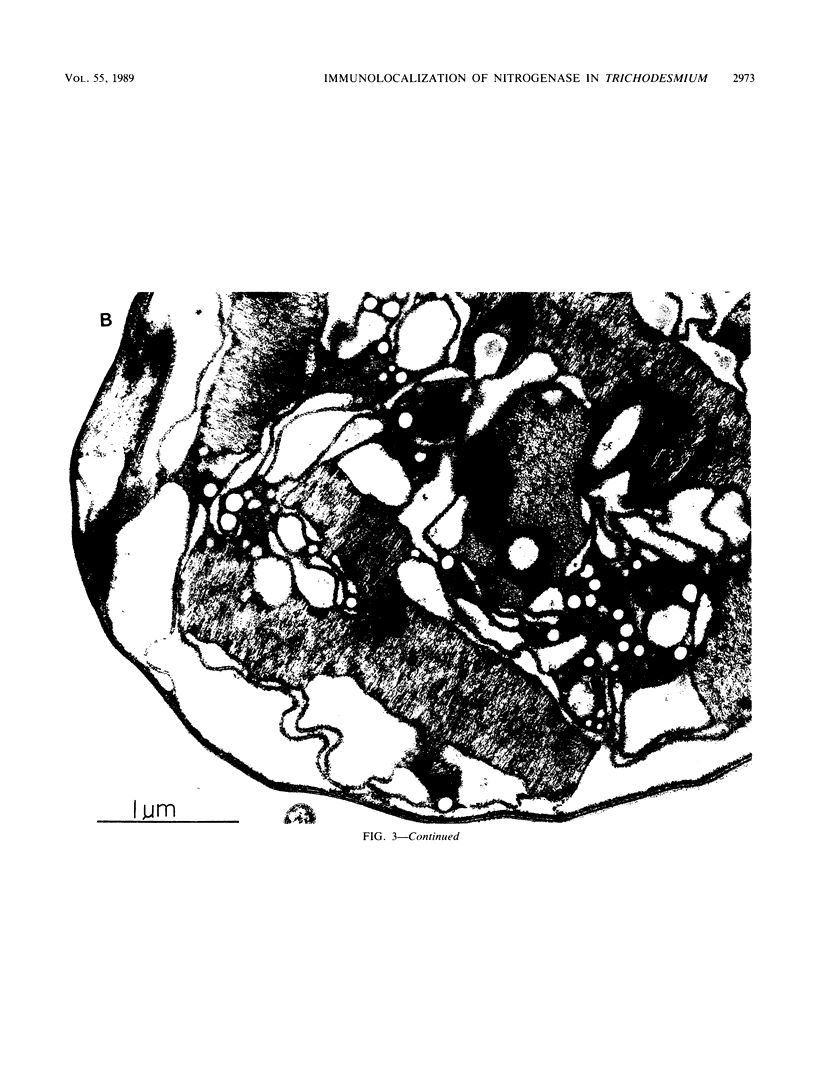
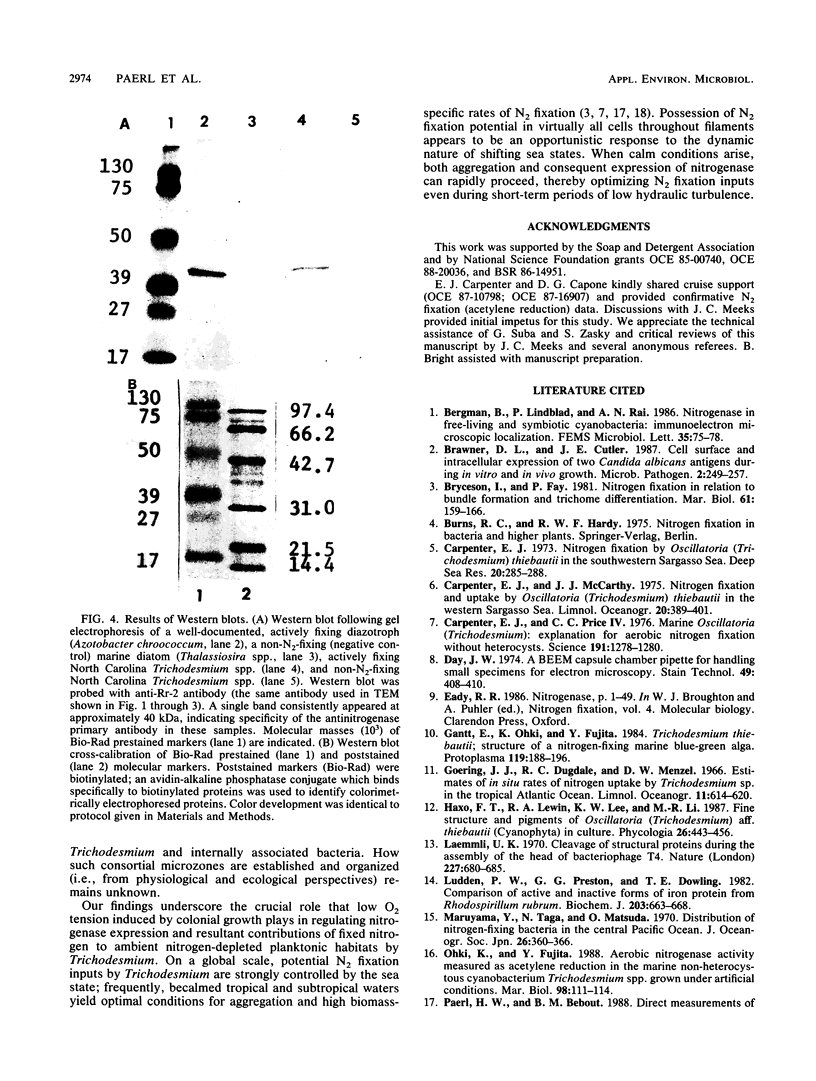
Abstract
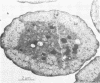
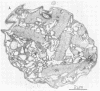
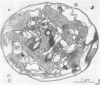
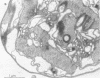
Colonial aggregation among nonheterocystous filaments of the planktonic marine cyanobacterium Trichodesmium is known to enhance N2 fixation, mediated by the O2-sensitive enzyme complex nitrogenase. Expression of nitrogenase appears linked to the formation of O2-depleted microzones within aggregated bacterium-associated colonies. While this implies a mechanism by which nonheterocystous N2 fixation can take place in an oxygenated water column, both the location and regulation of the N2-fixing apparatus remain unknown. We used an antinitrogenase polyclonal antibody together with postsection immunocolloidal gold staining and transmission electron microscopy to show that (i) virtually all Trichodesmium cells within a colony possessed nitrogenase, (ii) nitrogenase showed no clear intracellular localization, and (iii) certain associated bacteria contained nitrogenase. Our findings emphasize the critical role coloniality plays in regulating nitrogenase expression in nature. We interpret the potential for a large share of Trichodesmium cells to fix N2 as an opportunistic response to the dynamic nature of the sea state; during quiescent conditions, aggregation and consequent expression of nitrogenase can proceed rapidly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawner D. L., Cutler J. E. Cell surface and intracellular expression of two Candida albicans antigens during in vitro and in vivo growth. Microb Pathog. 1987 Apr;2(4):249–257. doi: 10.1016/0882-4010(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Day J. W. A BEEM capsule chamber-pipette for handling small specimens for electron microscopy. Stain Technol. 1974 Nov;49(6):408–410. doi: 10.3109/10520297409117019. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Preston G. G., Dowling T. E. Comparison of active and inactive forms of iron protein from Rhodospirillum rubrum. Biochem J. 1982 Jun 1;203(3):663–668. doi: 10.1042/bj2030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Bland P. T. Localized Tetrazolium Reduction in Relation to N(2) Fixation, CO(2) Fixation, and H(2) Uptake in Aquatic Filamentous Cyanobacteria. Appl Environ Microbiol. 1982 Jan;43(1):218–226. doi: 10.1128/aem.43.1.218-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baalen C., Brown R. M., Jr The ultrastructure of the marine blue green alga, Trichodesmium erythraeum, with special reference to the cell wall, gas vacuoles, and cylindrical bodies. Arch Mikrobiol. 1969;69(1):79–91. doi: 10.1007/BF00408566. [DOI] [PubMed] [Google Scholar]