Abstract
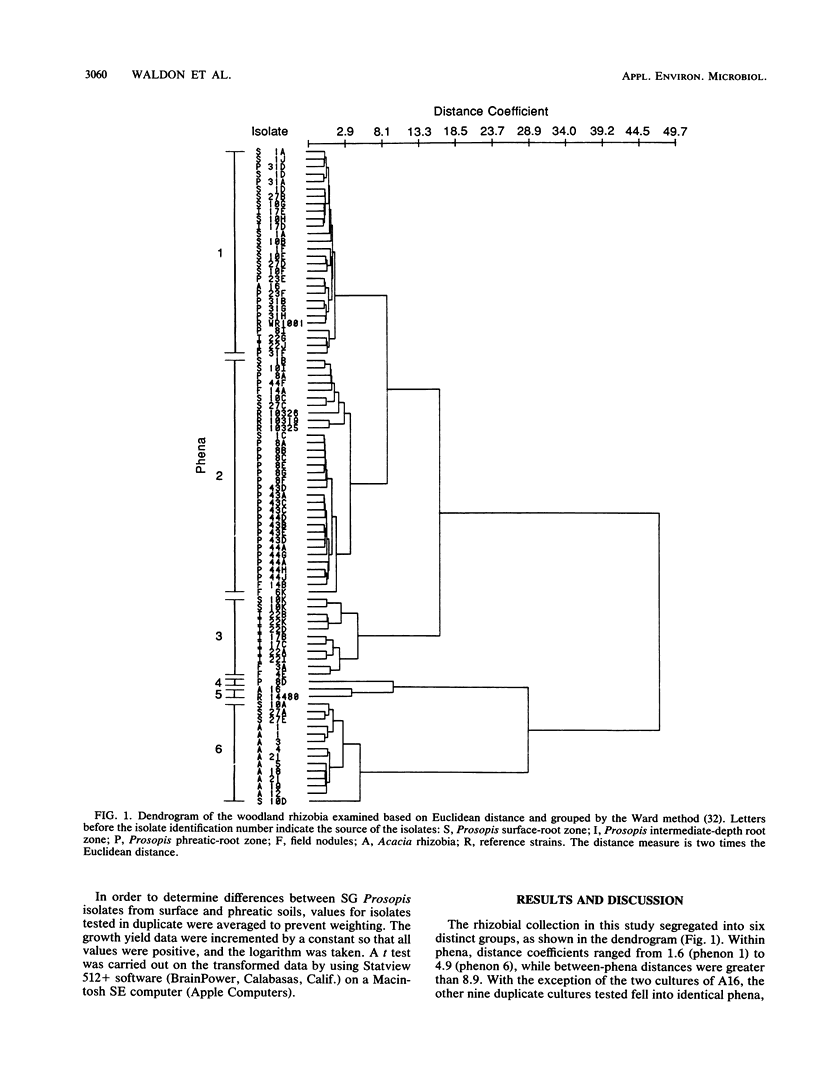
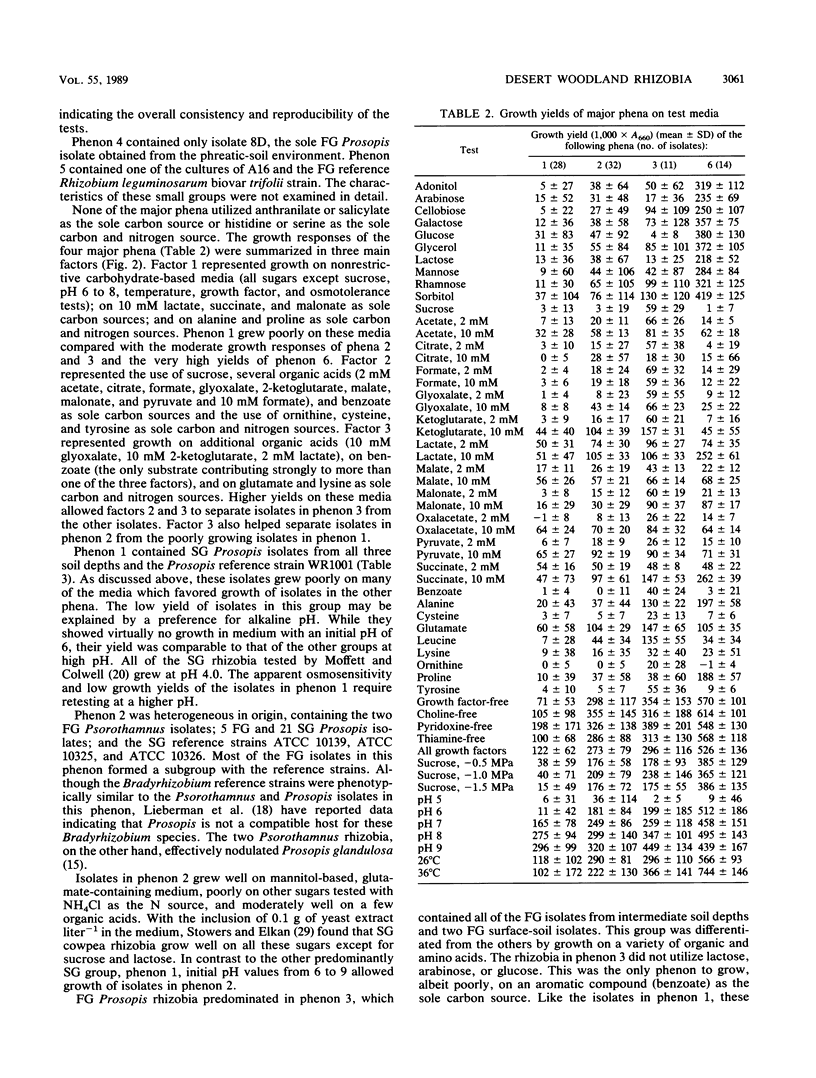
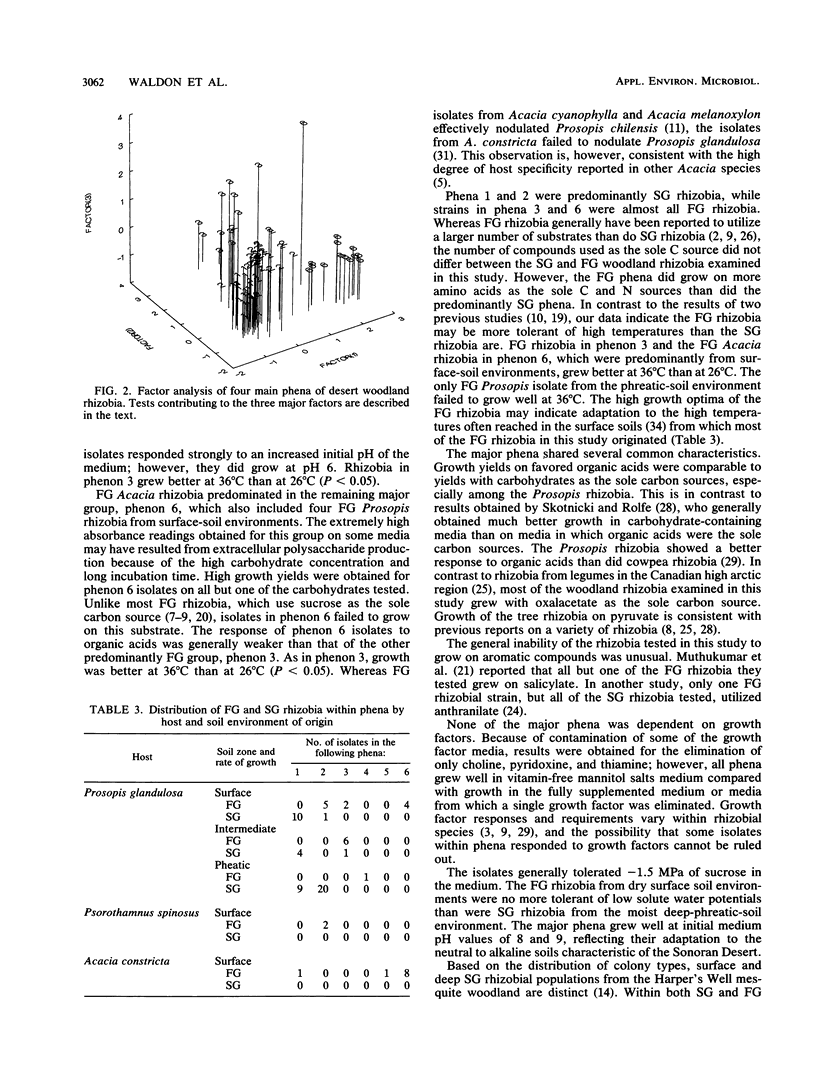
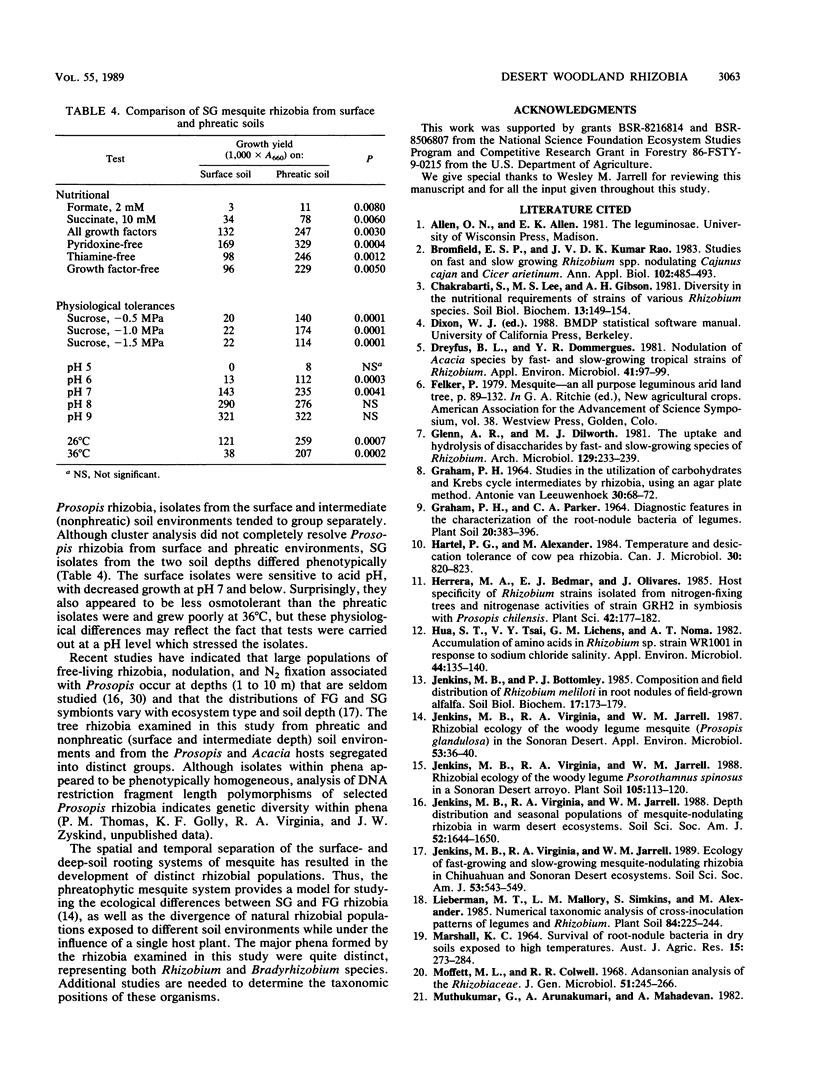
A collection of 74 rhizobial isolates recovered from nodules of the desert woody legumes Prosopis glandulosa, Psorothamnus spinosus, and Acacia constricta were characterized by using 61 nutritional and biochemical tests. We compared isolates from A. constricta and Prosopis glandulosa and tested the hypothesis that the rhizobia from a deep-phreatic rooting zone of a Prosopis woodland in the Sonoran Desert of southern California were phenetically distinct from rhizobia from surface soils. Cluster analysis identified four major homogeneous groups. The first phenon contained slow-growing (SG) Prosopis rhizobia from surface and deep-phreatic-soil environments. These isolates grew poorly on most of the media used in the study, probably because of their requirement for a high medium pH. The second group of isolates primarily contained SG Prosopis rhizobia from the deep-phreatic rooting environment and included two fast-growing (FG) Psorothamnus rhizobia. These isolates were nutritionally versatile and grew over a broad pH range. The third major phenon was composed mainly of FG Prosopis rhizobia from surface and dry subsurface soils. While these isolates used a restricted range of carbohydrates (including sucrose) as sole carbon sources, they showed better growth on a range of organic acids as sole carbon sources and amino acids as sole carbon and nitrogen sources than did other isolates in the study. They grew better at 36°C than at 26°C. The FG Acacia rhizobia from surface-soil environments formed a final major phenon that was distinct from the Prosopis isolates. They produced very high absorbance readings on all of the carbohydrates tested except sucrose, grew poorly on many of the other substrates tested, and preferred a 36 to a 26°C incubation temperature. The surface populations of Prosopis rhizobia required a higher pH for growth and, under the conditions used in this study, were less tolerant of low solute potential and high growth temperature than were phreatic-soil isolates. SG Prosopis rhizobia from phreatic and surface soils were physiologically distinct, suggesting adaptation to their respective soil environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreyfus B. L., Dommergues Y. R. Nodulation of acacia species by fast- and slow-growing tropical strains of Rhizobium. Appl Environ Microbiol. 1981 Jan;41(1):97–99. doi: 10.1128/aem.41.1.97-99.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM P. H. STUDIES ON THE UTILISATION OF CARBOHYDRATES AND KREBS CYCLE INTERMEDIATES BY RHIZOBIA, USING AN AGAR PLATE METHOD. Antonie Van Leeuwenhoek. 1964;30:68–72. doi: 10.1007/BF02046703. [DOI] [PubMed] [Google Scholar]
- Hua S. S., Tsai V. Y., Lichens G. M., Noma A. T. Accumulation of Amino Acids in Rhizobium sp. Strain WR1001 in Response to Sodium Chloride Salinity. Appl Environ Microbiol. 1982 Jul;44(1):135–140. doi: 10.1128/aem.44.1.135-140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett M. L., Colwell R. R. Adansonian analysis of the Rhizobiaceae. J Gen Microbiol. 1968 Apr;51(2):245–266. doi: 10.1099/00221287-51-2-245. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Rolfe B. G. Differential stimulation and inhibition of growth of Rhizobium trifolii strain T1 and other Rhizobium species by various carbon sources. Microbios. 1977;20(79):15–28. [PubMed] [Google Scholar]


