Abstract
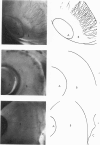
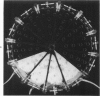
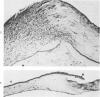
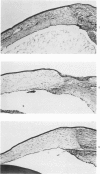
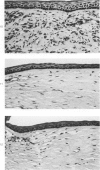
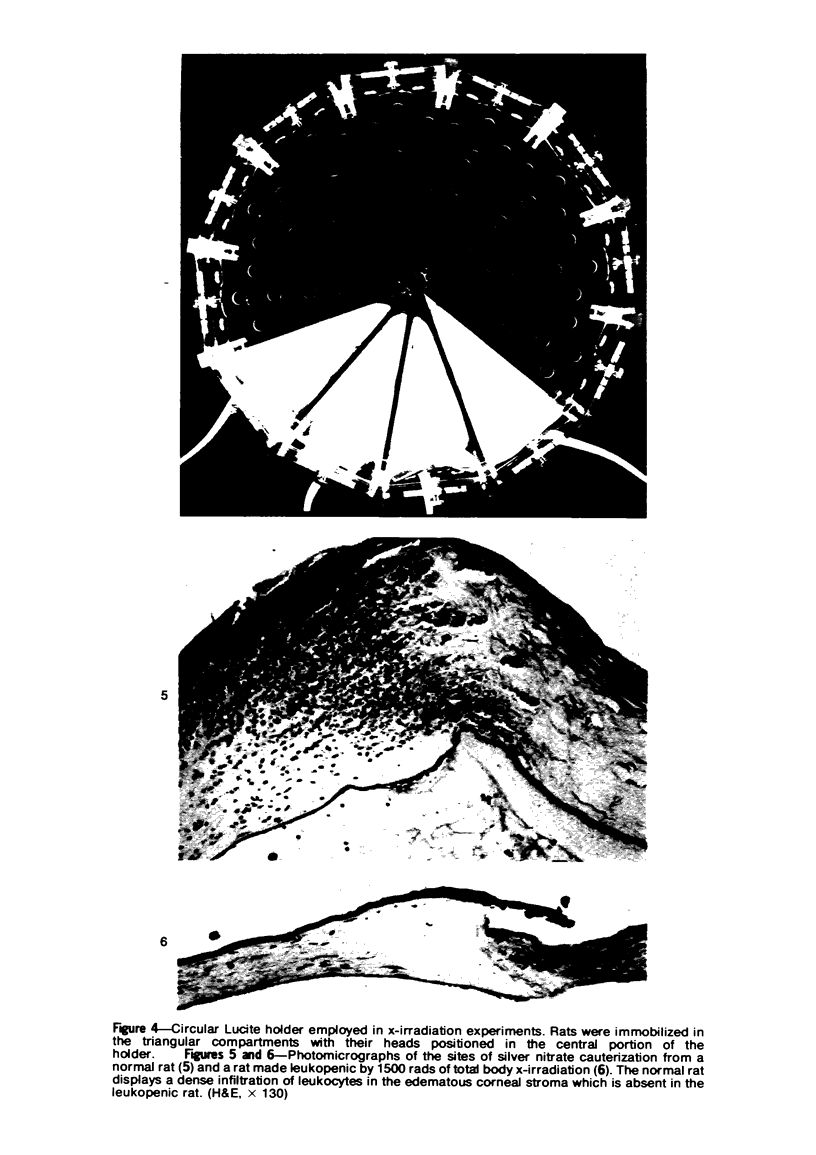
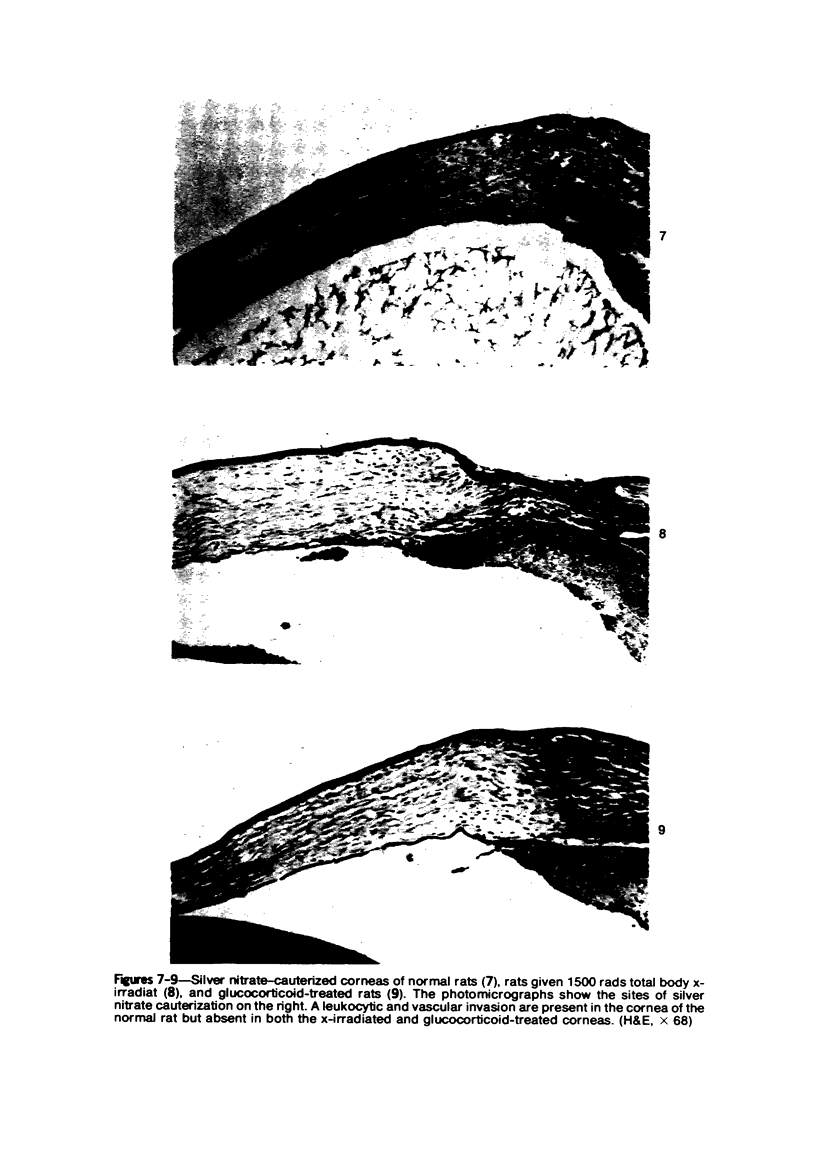
Investigations on several experimental models in the past have supported the hypotheses that corneal vascularization is a manifestation of the inflammatory response and that leukocytes perform an essential role in stimulating corneal vascular ingrowth. To evaluate the possible role of leukocytes further in this phenomenon, the effect of leukocyte elimination on corneal vascularization induced by silver nitrate cauterization was investigated. Weanling Fischer albino rats received doses of total body x-irradiation ranging from 1100 to 2100 rads to deplete circulating leukocytes, and corneal silver nitrate cauterization was performed 4 days later. In this model, animals that received 1500 rads or more total body x-irradiation became severely leukopenic within 4 days. As a rule, neither leukocytes nor blood vessels invaded the cauterized corneas, whereas both a leukocytic and vascular invasion occurred at lower doses of irradiation that did not totally eliminate circulating leukocytes. Corneal vascularization ensued if the corneal cauterization was performed immediately after total body x-irradiation with 1500 rads before the leukopenic effect of x-irradiation occurred. Control studies in which the cornea was cauterized 4 days after only the head received 1500 rads x-irradiation ruled out the possibility of irradiation-induced limbal endothelial damage as the explanation for the vascular suppression observed by x-ray treatment. In nonirradiated rats, silver nitrate cauterization of the cornea consistently induced corneal vascularization by 2 to 3 days. In further experiments, methylprednisolone acetate was administered subconjunctivally after corneal cauterization. This corticosteroid inhibited the infiltration of leukocytes and the subsequent vascular invasion into the corneal stroma, if administered immediately after silver nitrate cauterization. However, when the same glucocorticoid was administered 1 day after cauterization, both a leukocytic infiltration and vascular ingrowth occurred but to a less severe degree than in non-glucocorticoid-treated cauterized corneas. These investigations together demonstrated that a vascular ingrowth of the cornea did not follow corneal cauterization with silver nitrate in the absence of leukocytes, and gives further support to the hypothesis that leukocytes serve a crucial function in corneal vascularization.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., COOK C. In vivo observations of the effects of cortisone upon the blood vessels in rabbits ear chambers. Br J Exp Pathol. 1952 Oct;33(5):445–450. [PMC free article] [PubMed] [Google Scholar]
- ASHTON N., COOK C., LANGHAM M. Effect of cortisone on vascularization and opacification of the cornea induced by alloxan. Br J Ophthalmol. 1951 Nov;35(11):718–724. doi: 10.1136/bjo.35.11.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHTON N., COOK C. Mechanism of corneal vascularization. Br J Ophthalmol. 1953 Apr;37(4):193–209. doi: 10.1136/bjo.37.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausprunk D. H., Knighton D. R., Folkman J. Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Role of host and preexisting graft blood vessels. Am J Pathol. 1975 Jun;79(3):597–618. [PMC free article] [PubMed] [Google Scholar]
- Burton A. F., Storr J. M., Dunn W. L. Cytolytic action of corticosteroids on thymus and lymphoma cells in vitro. Can J Biochem. 1967 Feb;45(2):289–297. doi: 10.1139/o67-032. [DOI] [PubMed] [Google Scholar]
- CAMPBELL F. W., MICHAELSON I. C. Blood-vessel formation in the cornea. Br J Ophthalmol. 1949 Apr;33(4):248–255. doi: 10.1136/bjo.33.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey R. C., Hughes W. F., Bloome M. A., Tallman C. B. Prevention of corneal vascularization. Am J Ophthalmol. 1968 Dec;66(6):1118–1131. doi: 10.1016/0002-9394(68)90821-0. [DOI] [PubMed] [Google Scholar]
- Folca P. J. Corneal vascularization induced experimentally with corneal extracts. Br J Ophthalmol. 1969 Dec;53(12):827–832. doi: 10.1136/bjo.53.12.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer C. H., Klintworth G. K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. Am J Pathol. 1975 Jun;79(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- GLASER R. J., BERRY J. W., LOEB L. H., WOOD W. B., Jr The effect of cortisone in streptococcal lymphadenitis and pneumonia. J Lab Clin Med. 1951 Sep;38(3):363–373. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- HULSE E. V. Lymphocyte depletion of the blood and bone marrow of the irradiated rat. A quantitative study. Br J Haematol. 1959 Jul;5:278–283. doi: 10.1111/j.1365-2141.1959.tb04035.x. [DOI] [PubMed] [Google Scholar]
- JONES I. S., MEYER K. Inhibition of vascularization of the rabbit cornea by local application of cortisone. Proc Soc Exp Biol Med. 1950 May;74(1):102–104. doi: 10.3181/00379727-74-17822. [DOI] [PubMed] [Google Scholar]
- KATZIN H. M., LA TESSA A. J., POLATNICK J. Possible antivascularization factors in the cornea. Am J Ophthalmol. 1956 Dec;42(6):897–899. doi: 10.1016/0002-9394(56)90660-2. [DOI] [PubMed] [Google Scholar]
- KETCHEL M. M., FAVOUR C. B., STURGIS S. H. The in vitro action of hydrocortisone on leucocyte migration. J Exp Med. 1958 Feb 1;107(2):211–218. doi: 10.1084/jem.107.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGHAM M. Observations on the growth of blood vessels into the cornea; application of a new experimental technique. Br J Ophthalmol. 1953 Apr;37(4):210–222. doi: 10.1136/bjo.37.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVERGNE G., COLMANT I. A. COMPARATIVE STUDY OF THE ACTION OF THIOTEPA AND TRIAMCINOLONE ON CORNEAL VASCULARIZATION IN RABBITS. Br J Ophthalmol. 1964 Aug;48:416–422. doi: 10.1136/bjo.48.8.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTER A., GREAVES D. P. Effect of cortisone upon the vascularization which follows corneal burns. 1. Heat burns. Br J Ophthalmol. 1951 Nov;35(11):725–729. doi: 10.1136/bjo.35.11.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Leibowitz H. M., Lass J. H., Kupferman A. Quantitation of inflammation in the cornea. Arch Ophthalmol. 1974 Nov;92(5):427–430. doi: 10.1001/archopht.1974.01010010439014. [DOI] [PubMed] [Google Scholar]
- MAYER G., MICHAELSON I. C., HERZ N. Hyaluronidase in ocular tissues. II. Hyaluronidase in the tissues of the rabbit's eye. Br J Ophthalmol. 1956 Jan;40(1):53–56. doi: 10.1136/bjo.40.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAELSON I. C. Effect of cortisone upon corneal vascularization produced experimentally. AMA Arch Ophthalmol. 1952 Apr;47(4):459–464. doi: 10.1001/archopht.1952.01700030469007. [DOI] [PubMed] [Google Scholar]
- Maurice D. M., Zauberman H., Michaelson I. C. The stimulus to neovascularization in the cornea. Exp Eye Res. 1966 Jul;5(3):168–184. doi: 10.1016/s0014-4835(66)80004-0. [DOI] [PubMed] [Google Scholar]
- PATT H. M., MALONEY M. A. A comparison of radiation-induced granulocytopenia in several mammalian species. Radiat Res. 1963 Feb;18:231–235. [PubMed] [Google Scholar]
- Polack F. M. Lymphocyte destruction during corneal homograft reaction. A scanning electron microscopic study. Arch Ophthalmol. 1973 May;89(5):413–416. doi: 10.1001/archopht.1973.01000040415012. [DOI] [PubMed] [Google Scholar]
- SCHREK R. Qualitative and quantitative reactions of lymphocytes to x rays. Ann N Y Acad Sci. 1961 Nov 13;95:839–848. doi: 10.1111/j.1749-6632.1961.tb50080.x. [DOI] [PubMed] [Google Scholar]
- Simpson D. M., Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972 Aug;51(8):2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWELL O. A. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952 Oct;64(4):687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]