Abstract
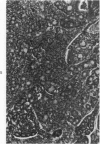
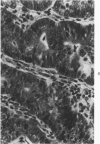
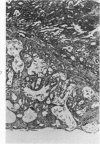
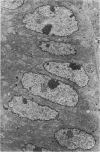
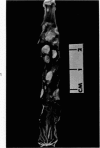
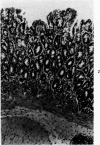
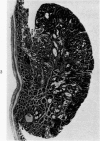
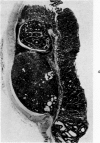
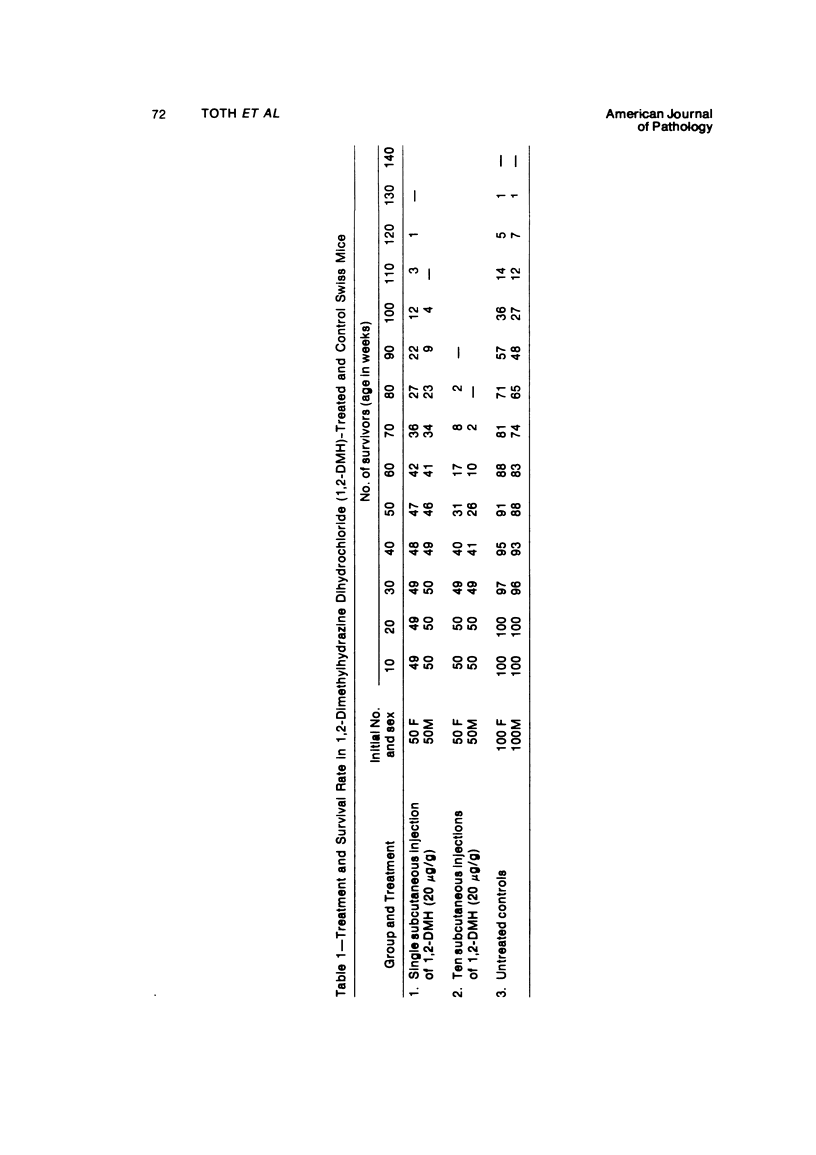
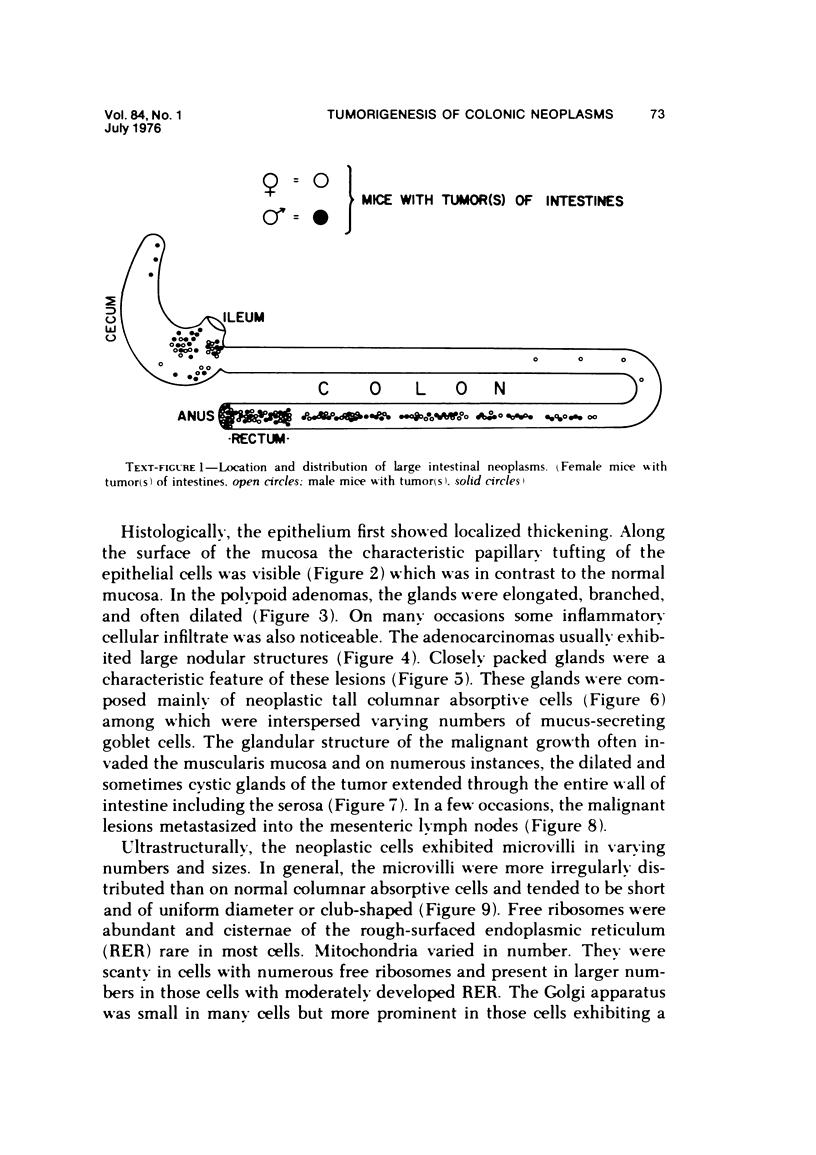
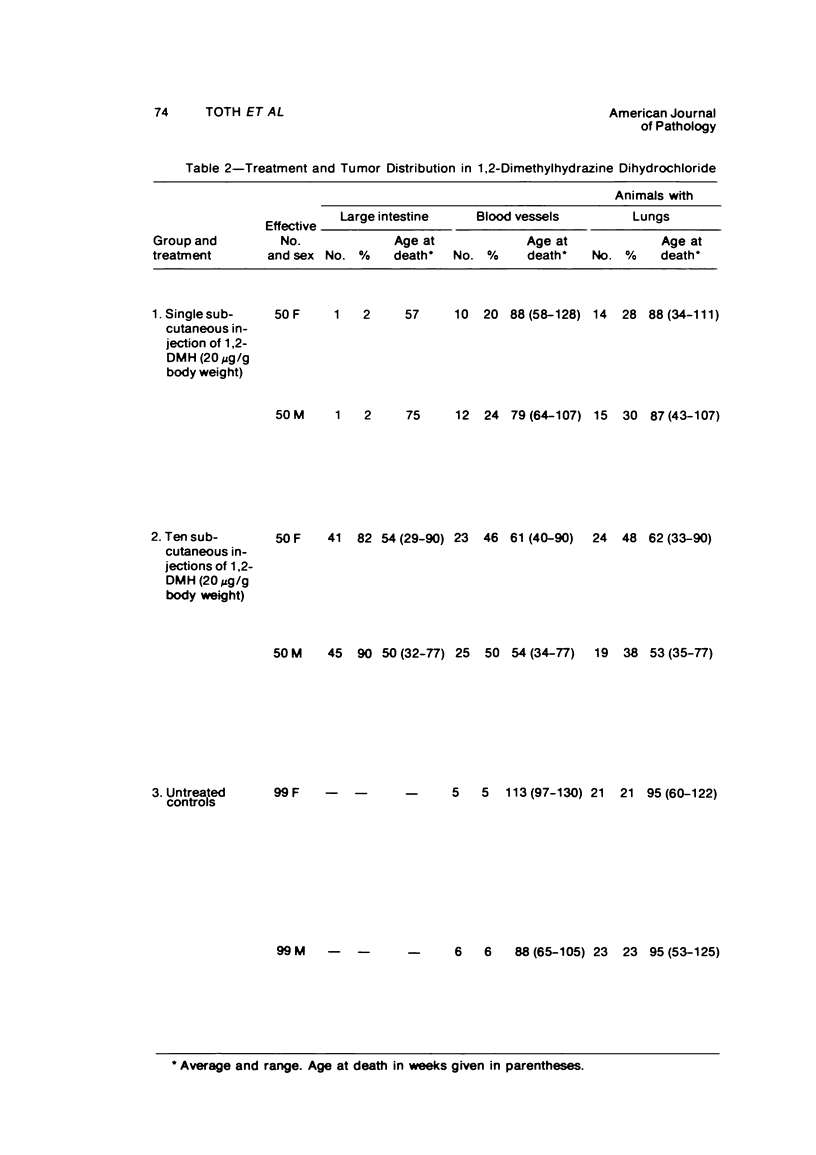
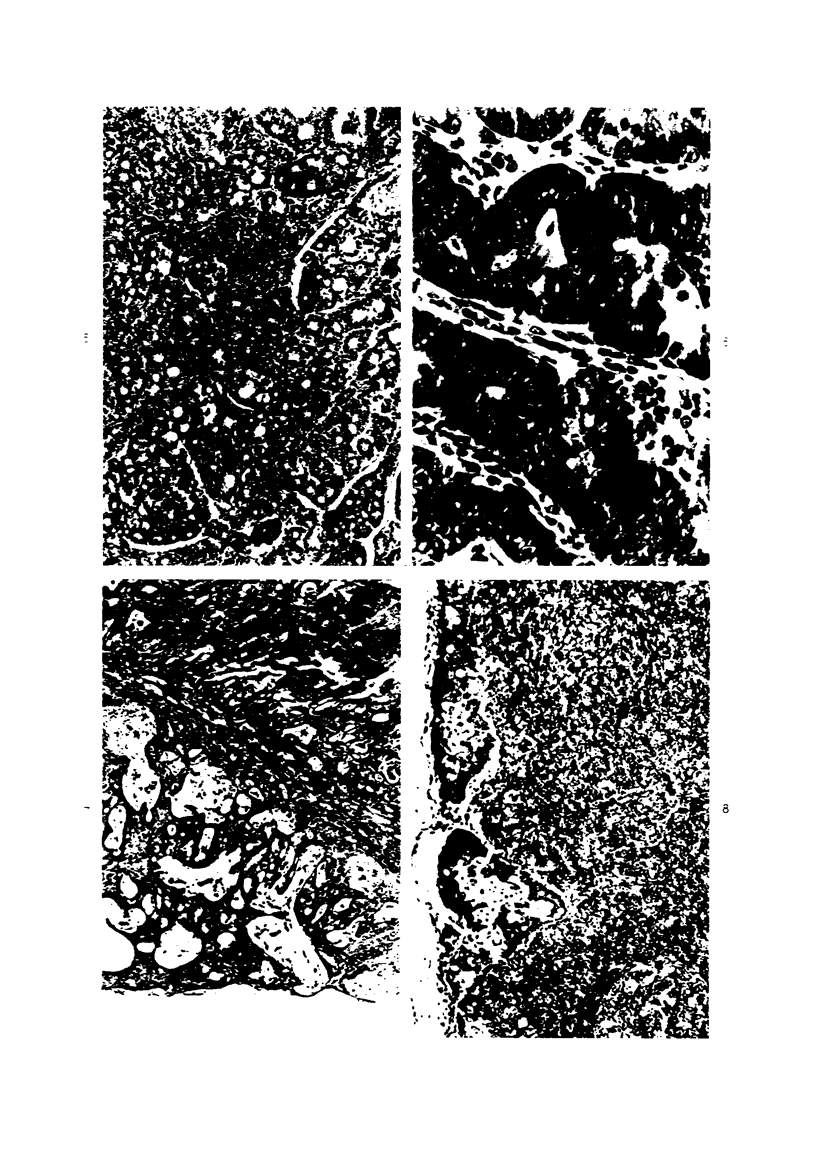
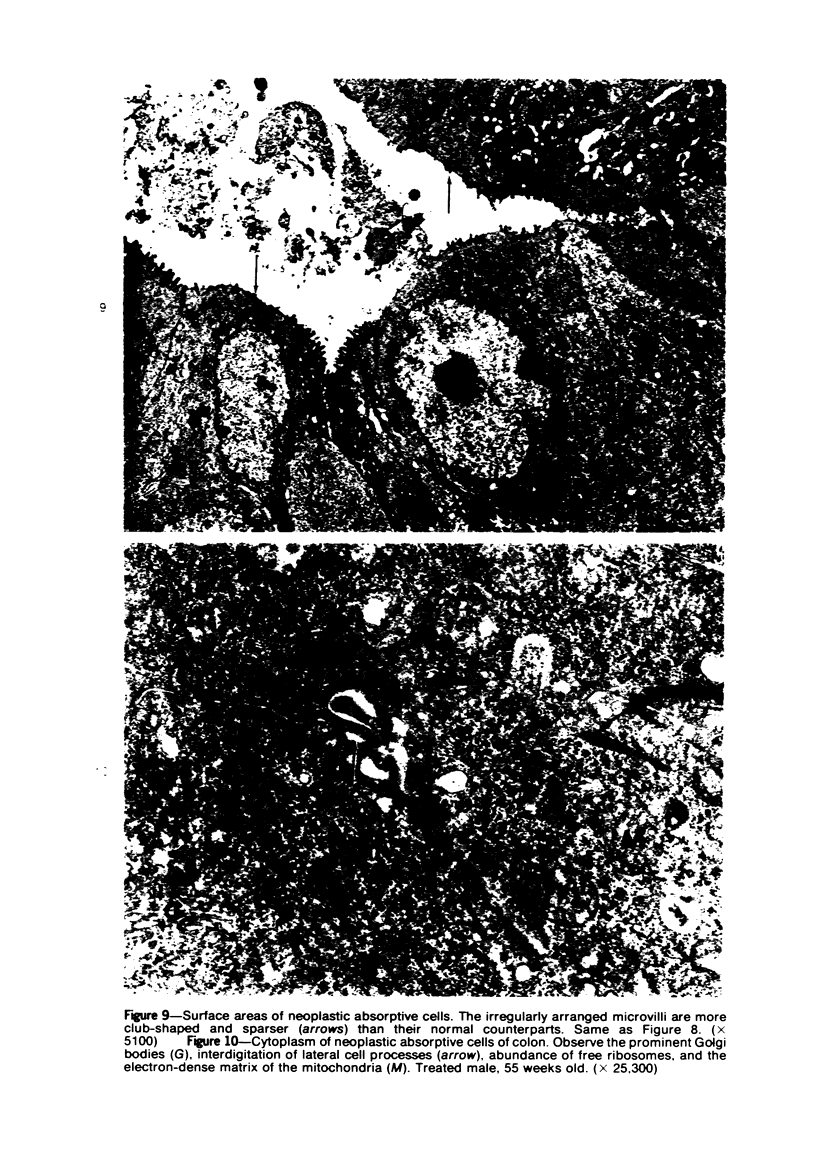
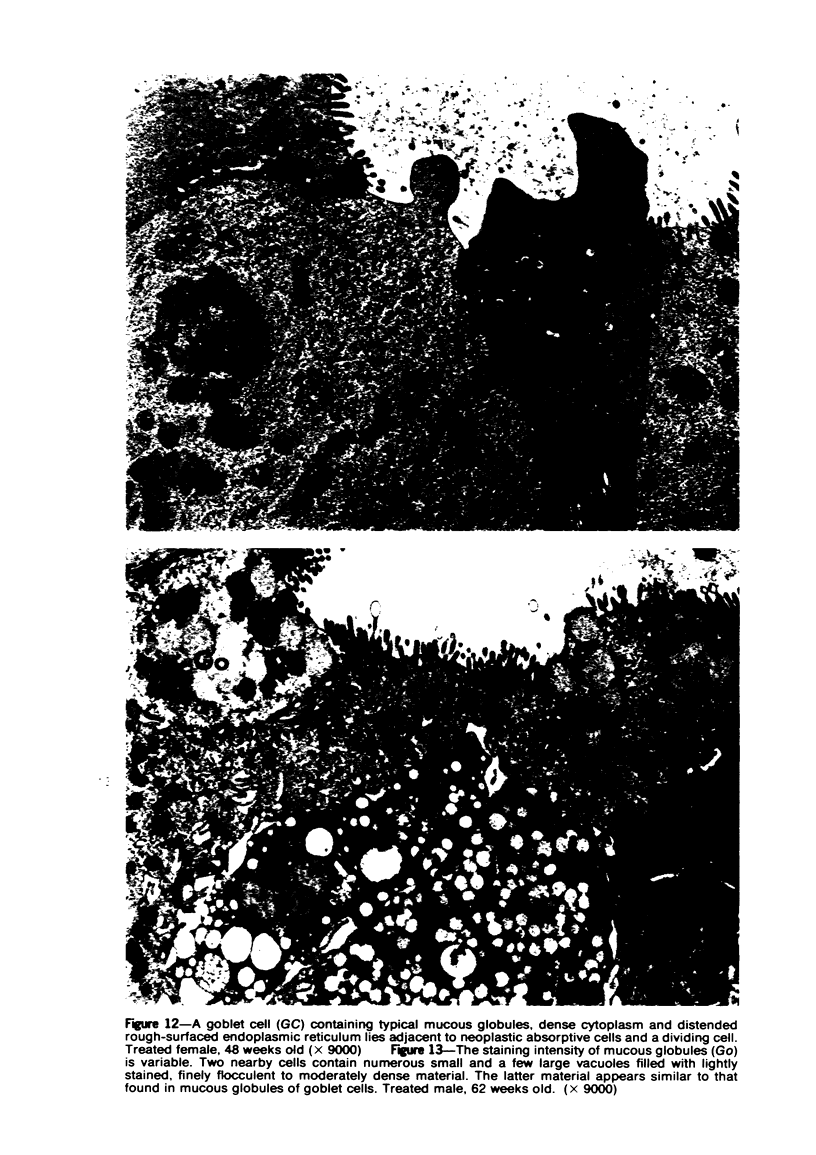
Single or ten weekly subcutaneous injection(s) of 1, 2-dimethylhydrazine dihydrochloride were administered separately to Swiss mice. The repeated application gave rise mainly to high incidences of tumors in the large intestine. These neoplasms occurred most frequently in the colorectal area and in cecum adjacent to ileum. Light microscopically, these lesions were classified as polypoid adenomas and adenocarcinomas. Most of the adenocarcinomas were highly invasive, although they metastasized rarely. The fine structure of the malignant cells exhibited features typical of columnar absorptive cells. A distinctive alteration was the disorderly arrangement and abnormal size and shape of the microvilli. In addition, the cells exhibited numerous free ribosomes, little RER, priminent Golgi bodies, and uniformly dispersed nuclear chromatin. Morphologically, the intestinal tumors were similar to those found in man. In addition, the repeated administration of 1, 2-DMH also induced significant incidences of neoplasms in blood vessels, lungs, anus, and kidneys while the single application produced tumors in blood vessels and liver. The main hypotheses attempting to explain the selective induction of large intestinal neoplasms are discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER E. R., SHARKEY D. A. The ultrastructure of colonic polyps and cancer with special reference to the epithelial inclusion bodies of Leuchtenberger. Cancer. 1962 Jan-Feb;15:160–170. doi: 10.1002/1097-0142(196201/02)15:1<160::aid-cncr2820150122>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Goldman H., Ming S., Hickock D. F. Nature and significance of hyperplastic polyps of the human colon. Arch Pathol. 1970 Apr;89(4):349–354. [PubMed] [Google Scholar]
- HAMPTON J. C. An electron microscopic study of mouse colon. Dis Colon Rectum. 1960 Sep-Oct;3:423–440. doi: 10.1007/BF02616806. [DOI] [PubMed] [Google Scholar]
- IMAI H., SAITO S., STEIN A. A. ULTRASTRUCTURE OF ADENOMATOUS POLYPS AND VILLOUS ADENOMAS OF THE LARGE INTESTINE. Gastroenterology. 1965 Feb;48:188–197. [PubMed] [Google Scholar]
- IMAI H., STEIN A. A. Ultrastructure of adenocarcinoma of the colon. Gastroenterology. 1963 Apr;44:410–418. [PubMed] [Google Scholar]
- Kaye G. I., Fenoglio C. M., Pascal R. R., Lane N. Comparative electron microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. Variations in cellular structure relative to the process of epithelial differentiation. Gastroenterology. 1973 May;64(5):926–945. [PubMed] [Google Scholar]
- Pittman F. E., Pittman J. C. An electron microscopic study of epithelium of normal human sigmoid colonic mucosa. Gut. 1966 Dec;7(6):644–661. doi: 10.1136/gut.7.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spjut H. J., Smith M. N. A comparative electron microscopic study of human and rat colonic polyps and carcinomas. Exp Mol Pathol. 1967 Feb;6(1):11–24. doi: 10.1016/0014-4800(67)90003-2. [DOI] [PubMed] [Google Scholar]
- TOTH B., MAGEE P. N., SHUBIK P. CARCINOGENESIS STUDY WITH DIMETHYLNITROSAMINE ADMINISTERED ORALLY TO ADULT AND SUBCUTANEOUSLY TO NEWBORN BALB-C MICE. Cancer Res. 1964 Nov;24:1712–1721. [PubMed] [Google Scholar]
- Toth B. 1,1-Dimethylhydrazine (unsymmetrical) carcinogenesis in mice. Light microscopic and ultrastructural studies on neoplastic blood vessels. J Natl Cancer Inst. 1973 Jan;50(1):181–194. doi: 10.1093/jnci/50.1.181. [DOI] [PubMed] [Google Scholar]
- Toth B. A toxicity method with calcium cyclamate for chronic carcinogenesis experiments. Tumori. 1972 May-Jun;58(3):136–141. [PubMed] [Google Scholar]
- Toth B., Shimizu H. 1-Carbamyl-2-phenylhydrazine tumorigenesis in Swiss mice. Morphology of lung adenomas. J Natl Cancer Inst. 1974 Jan;52(1):241–251. doi: 10.1093/jnci/52.1.241. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebecke B., Löhrs U., Gimmy J., Eder M. Erzeugung von Darmtumoren bei Mäusen durch 1,2-Dimethylhydrazin. Z Gesamte Exp Med. 1969;149(3):277–278. [PubMed] [Google Scholar]