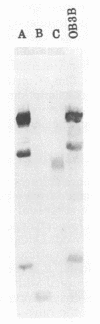
Abstract
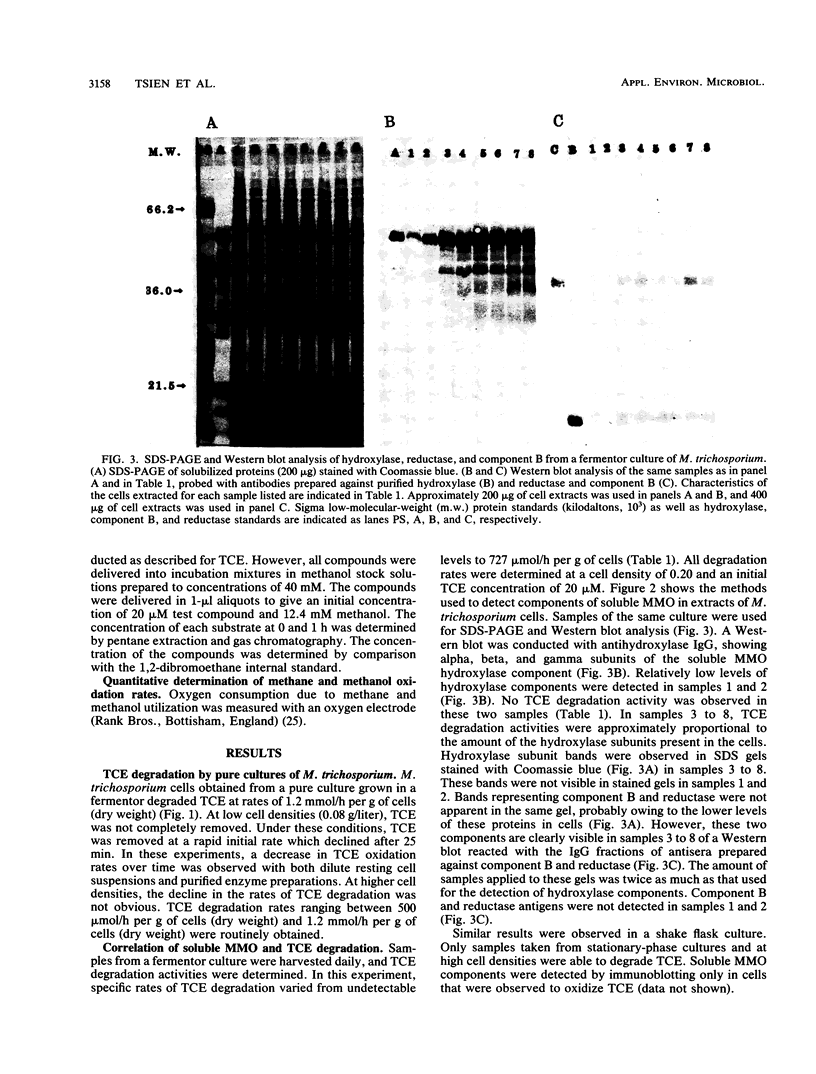
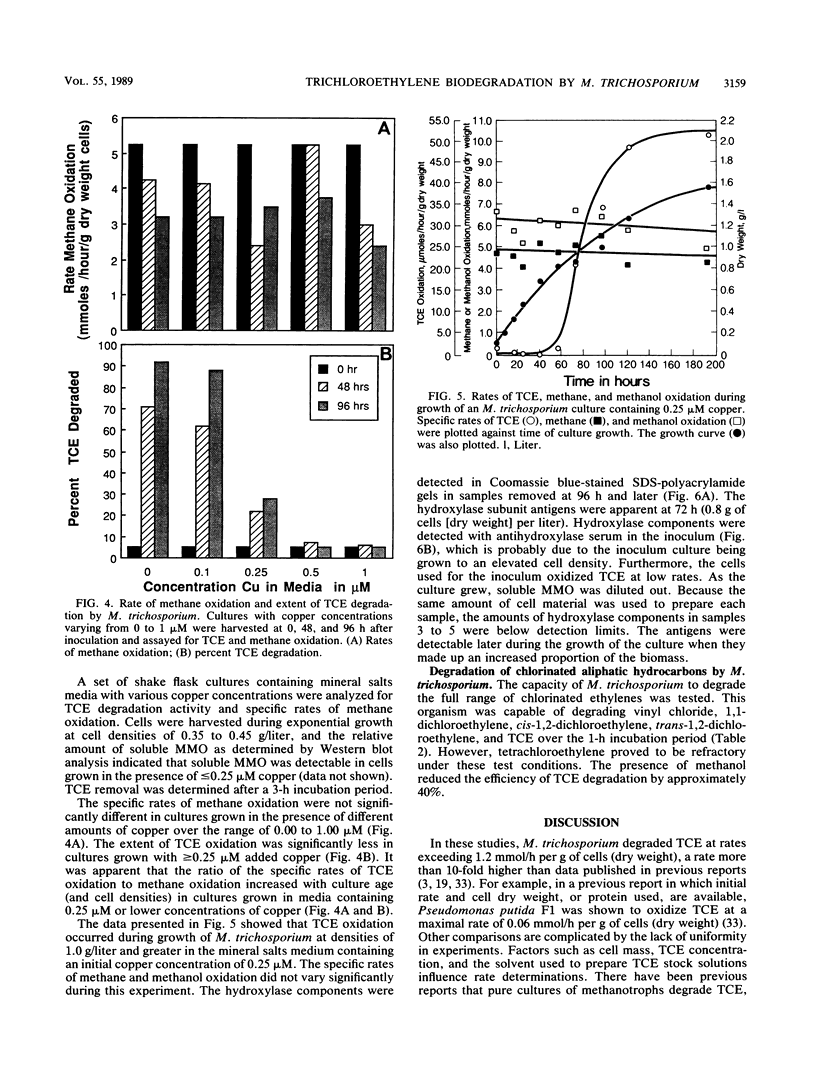
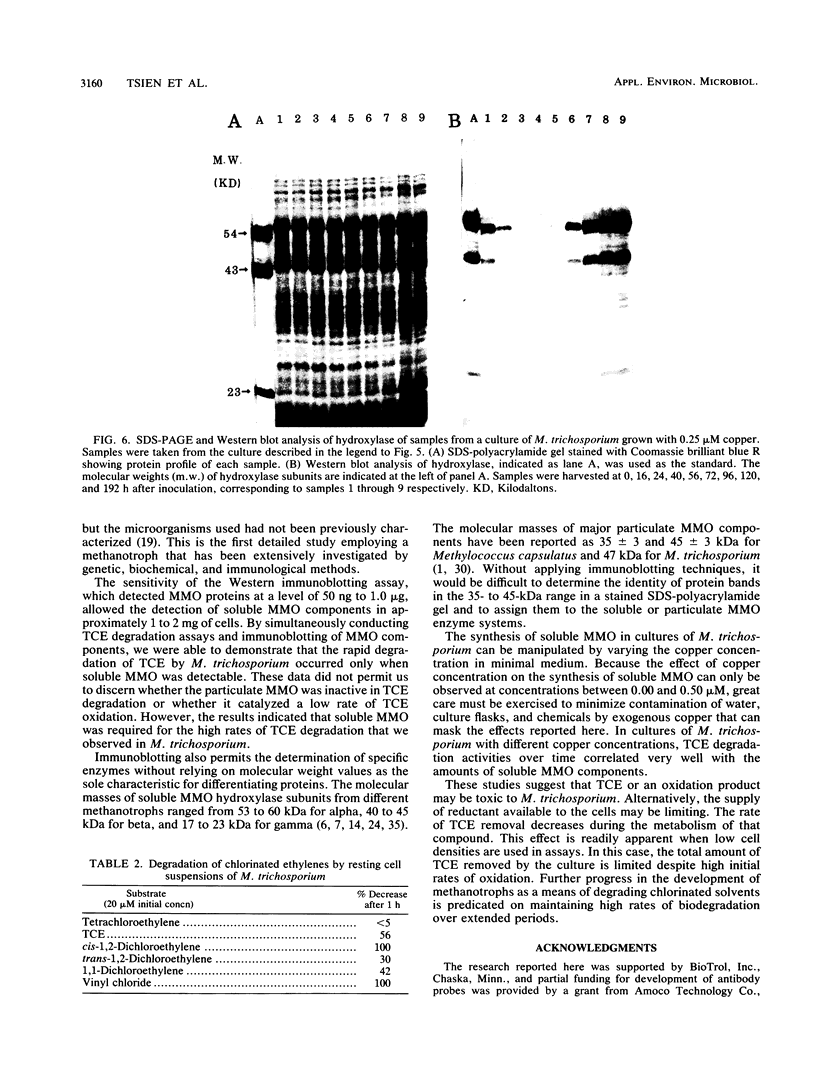
The methanotroph Methylosinus trichosporium OB3b, a type II methanotroph, degraded trichloroethylene at rates exceeding 1.2 mmol/h per g (dry weight) following the appearance of soluble methane monooxygenase in continuous and batch cultures. Cells capable oxidizing trichloroethylene contained components of soluble methane monooxygenase as demonstrated by Western blot (immunoblot) analysis with antibodies prepared against the purified enzyme. Growth of cultures in a medium containing 0.25 microM or less copper sulfate caused derepression of the synthesis of soluble methane monooxygenase. In these cultures, the specific rates of methane and methanol oxidation did not change during growth, while trichloroethylene oxidation increased with the appearance of soluble methane monooxygenase. M. trichosporium OB3b cells that contained soluble methane monooxygenase also degraded vinyl chloride, 1,1-dichloroethylene, cis-1,2-dichloroethylene, and trans-1,2-dichloroethylene.
Full text
PDF

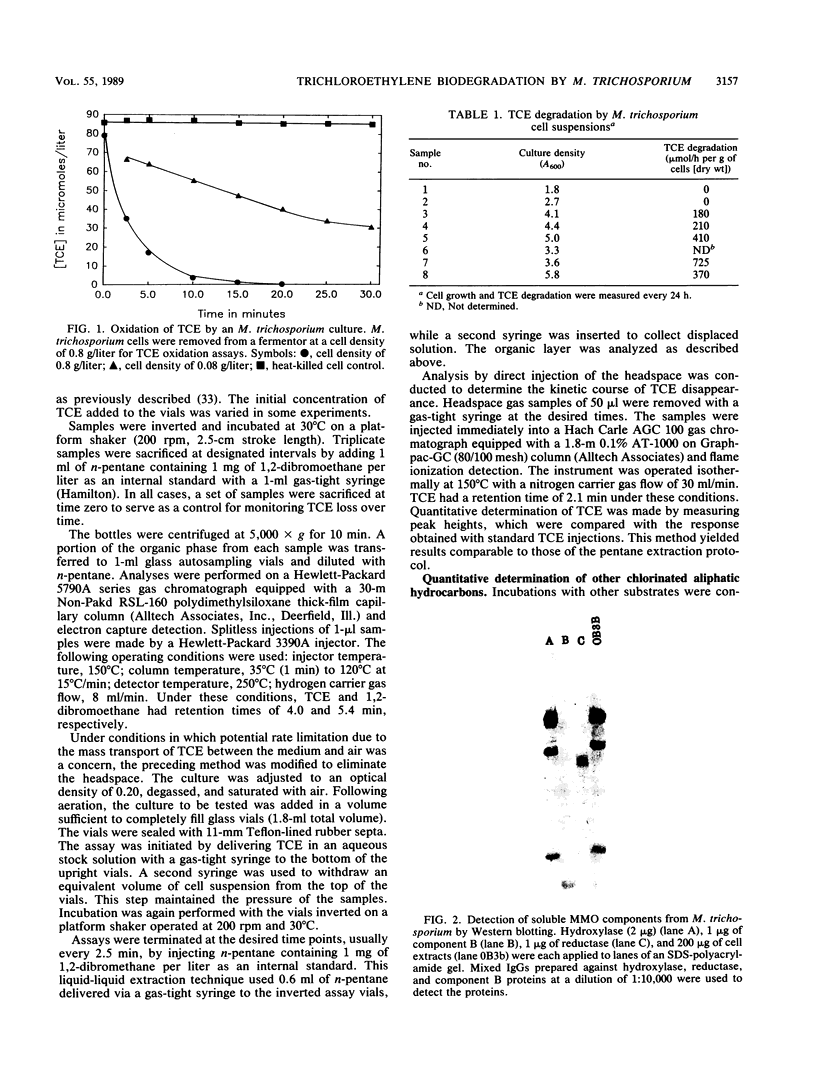

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arciero D., Vannelli T., Logan M., Hooper A. B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989 Mar 15;159(2):640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J. 1976 Aug 1;157(2):495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Phelps T. J., Ringelberg D., Mikell A. T., White D. C. Mineralization of trichloroethylene by heterotrophic enrichment cultures. Appl Environ Microbiol. 1988 Jul;54(7):1709–1714. doi: 10.2172/666263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M. M., Taddeo A. R., Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986 Apr;51(4):720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Froland W. A., Dege J. E., Lipscomb J. D. Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph. J Biol Chem. 1989 Jun 15;264(17):10023–10033. [PubMed] [Google Scholar]
- Fox B. G., Lipscomb J. D. Purification of a high specific activity methane monooxygenase hydroxylase component from a type II methanotroph. Biochem Biophys Res Commun. 1988 Jul 15;154(1):165–170. doi: 10.1016/0006-291x(88)90665-1. [DOI] [PubMed] [Google Scholar]
- Haber C. L., Allen L. N., Zhao S., Hanson R. S. Methylotrophic bacteria: biochemical diversity and genetics. Science. 1983 Sep 16;221(4616):1147–1153. doi: 10.1126/science.221.4616.1147. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C. New findings in methane-utilizing bacteria highlight their importance in the biosphere and their commercial potential. Nature. 1980 Aug 7;286(5773):561–564. doi: 10.1038/286561a0. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Hammond R. C., Sariaslani F. S., Best D., Davies M. M., Tryhorn S. E., Taylor F. Biotransformation of hydrocarbons and related compounds by whole organism suspensions of methane-grown methylosinus trichosporium OB 3b. Biochem Biophys Res Commun. 1979 Jul 27;89(2):671–677. doi: 10.1016/0006-291x(79)90682-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin S. M., Tam P. E., Bastien C. A., Hanson R. S. Genetic and physical analyses of Methylobacterium organophilum XX genes encoding methanol oxidation. J Bacteriol. 1988 Jan;170(1):141–148. doi: 10.1128/jb.170.1.141-148.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res. 1983 Mar;43(3):1145–1152. [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., O'neill E. J., Pritchard P. H. Aerobic metabolism of trichloroethylene by a bacterial isolate. Appl Environ Microbiol. 1986 Aug;52(2):383–384. doi: 10.1128/aem.52.2.383-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Savas J. C. Purification and properties of the hydroxylase component of methane monooxygenase. J Bacteriol. 1987 May;169(5):2313–2317. doi: 10.1128/jb.169.5.2313-2317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Schmidt E. L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977 Sep;23(9):1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Wilson B. H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985 Jan;49(1):242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland M. P., Dalton H. Purification and characterization of component A of the methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem. 1984 Jan 10;259(1):53–59. [PubMed] [Google Scholar]