Abstract
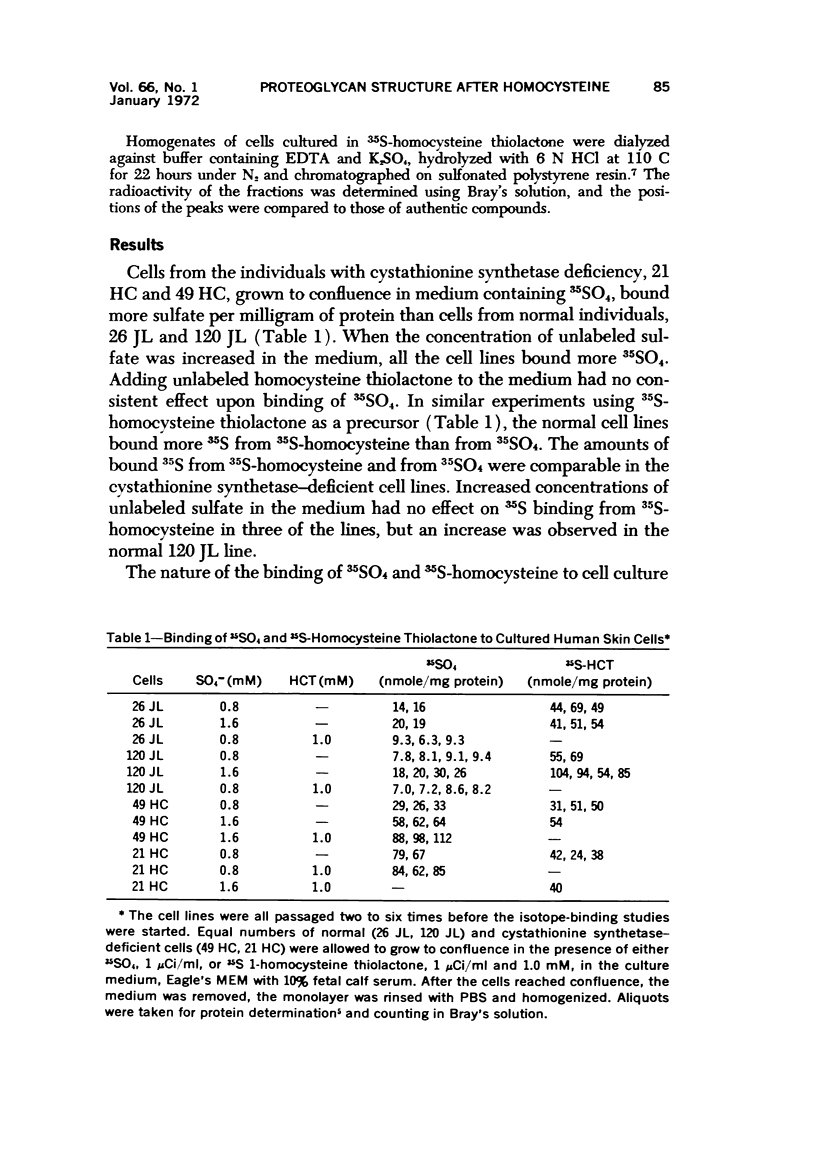
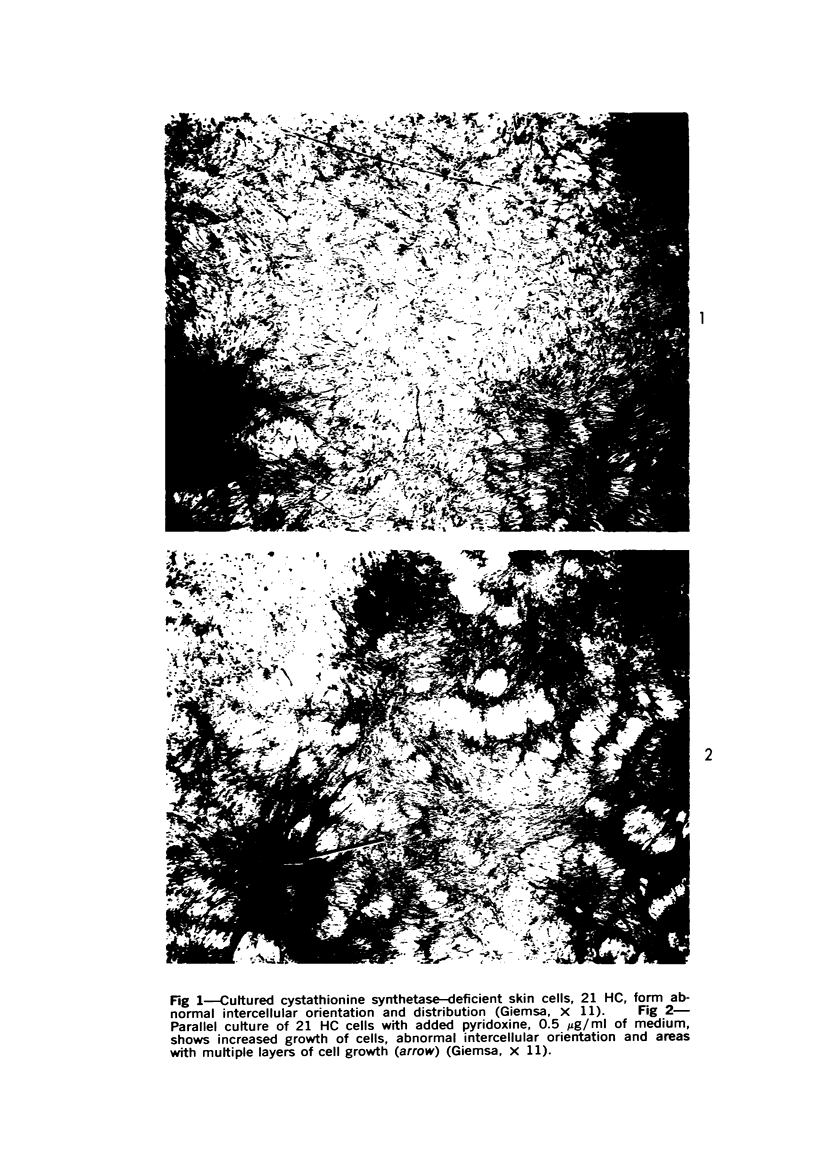
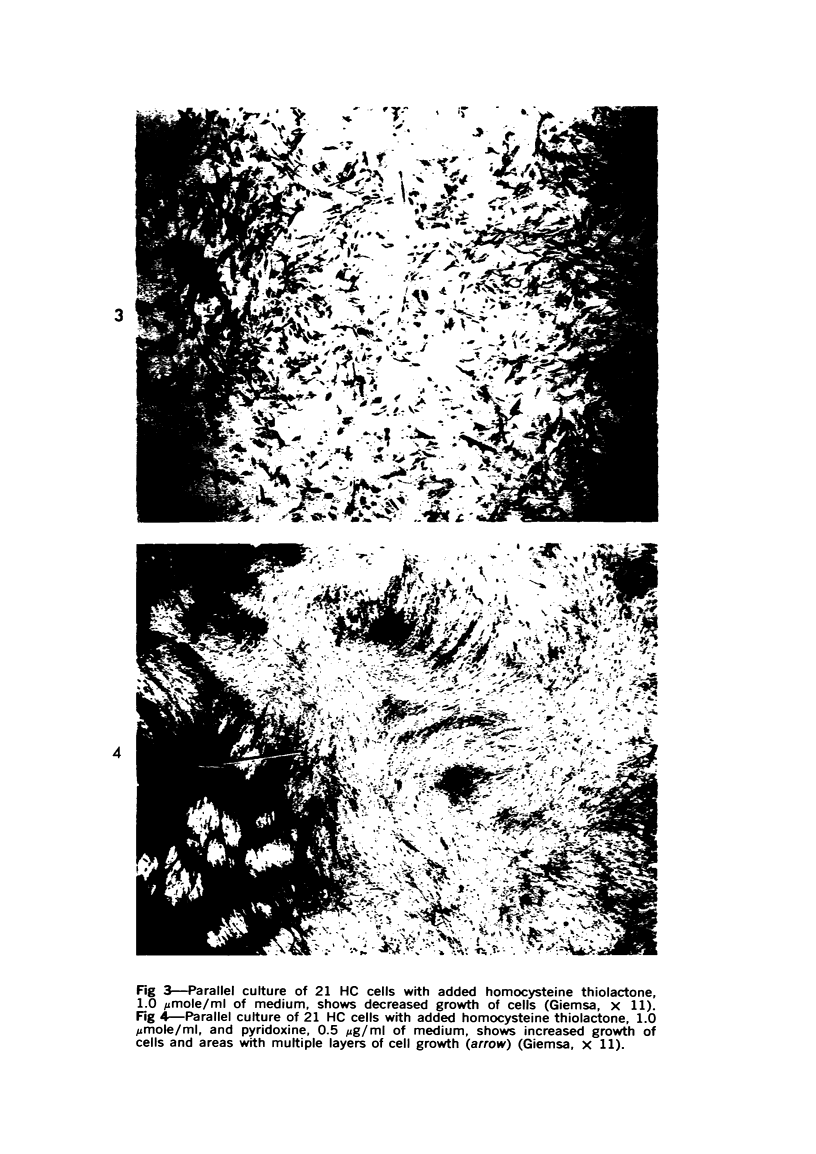
Cell culture monolayers deficient in cystathionine synthetase bound more inorganic sulfate than normal cell monolayers during growth to confluence; this was correlated with the production of granular proteoglycan by the abnormal cells and fibrillar proteoglycan by normal cells. Homocysteine was demonstrated to be an active precursor of esterified sulfate, confirming our previous finding of this sulfation pathway in liver. The cell cultures deficient in cystathionine synthetase were found to assume an abnormal cellular distribution on the surface of the culture dish, resembling the distribution assumed by neoplastic cells with loss of contact inhibition; the degree of abnormality of the cellular distribution was correlated with the amount of granular proteoglycan produced by the cells and the amount of inorganic sulfate binding by the cell monolayers. Pyridoxine was found to increase the growth rate of cell cultures from a patient with pyridoxineresponsive homocystinuria and to increase the production of fibrillar proteoglycan by the cells; no effect of pyridoxine was observed in the cell cultures from a patient who failed to respond to pyridoxine therapy. The findings suggest that the change in macromolecular conformation of cellular proteoglycans from fibrillar to granular is due to increased sulfation of the carbohydrate envelope of the molecule. The significance of the findings is related to the pathogenesis of homocystinuria, the phenomenon of contact inhibition, the action of growth hormone and initiation of arteriosclerotic plaques.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E., KARTHAUSER H. M. Social behaviour of cells in tissue culture. III. Mutual influence of sarcoma cells and fibroblasts. Exp Cell Res. 1957 Oct;13(2):276–291. doi: 10.1016/0014-4827(57)90007-1. [DOI] [PubMed] [Google Scholar]
- CARSON N. A., DENT C. E., FIELD C. M., GAULL G. E. HOMOCYSTINURIA: CLINICAL AND PATHOLOGICAL REVIEW OF TEN CASES. J Pediatr. 1965 Mar;66:565–583. doi: 10.1016/s0022-3476(65)80121-4. [DOI] [PubMed] [Google Scholar]
- CURRAN R. C., CRANE W. A. Mucopolysaccharides in the atheromatous aorta. J Pathol Bacteriol. 1962 Oct;84:405–412. doi: 10.1002/path.1700840214. [DOI] [PubMed] [Google Scholar]
- GIBSON J. B., CARSON N. A., NEILL D. W. PATHOLOGICAL FINDINGS IN HOMOCYSTINURIA. J Clin Pathol. 1964 Jul;17:427–437. doi: 10.1136/jcp.17.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATSUO Y., GREENBERG D. M. Metabolic formation of homoserine and alpha-aminobutyric acid from methionine. J Biol Chem. 1955 Aug;215(2):547–554. [PubMed] [Google Scholar]
- McCully K. S. Homocysteine metabolism in scurvy, growth and arteriosclerosis. Nature. 1971 Jun 11;231(5302):391–392. doi: 10.1038/231391a0. [DOI] [PubMed] [Google Scholar]
- McCully K. S. Importance of homocysteine-induced abnormalities of proteoglycan structure in arteriosclerosis. Am J Pathol. 1970 Apr;59(1):181–194. [PMC free article] [PubMed] [Google Scholar]
- McCully K. S., Ragsdale B. D. Production of arteriosclerosis by homocysteinemia. Am J Pathol. 1970 Oct;61(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- McCully K. S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969 Jul;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Medes G., Floyd N. Metabolism of sulphur: Further investigation of the enzymic oxidation of sulphur-containing amino-acids. Biochem J. 1942 Feb;36(1-2):259–270. doi: 10.1042/bj0360259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]