Abstract
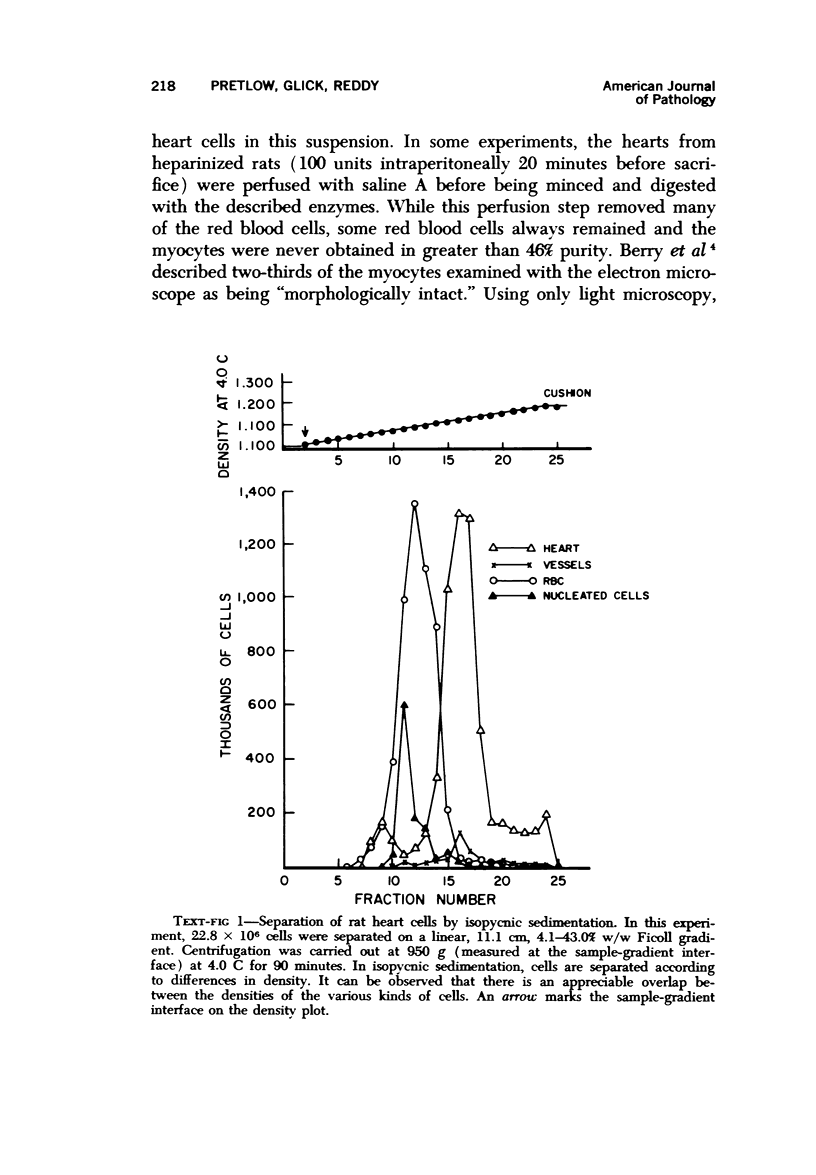
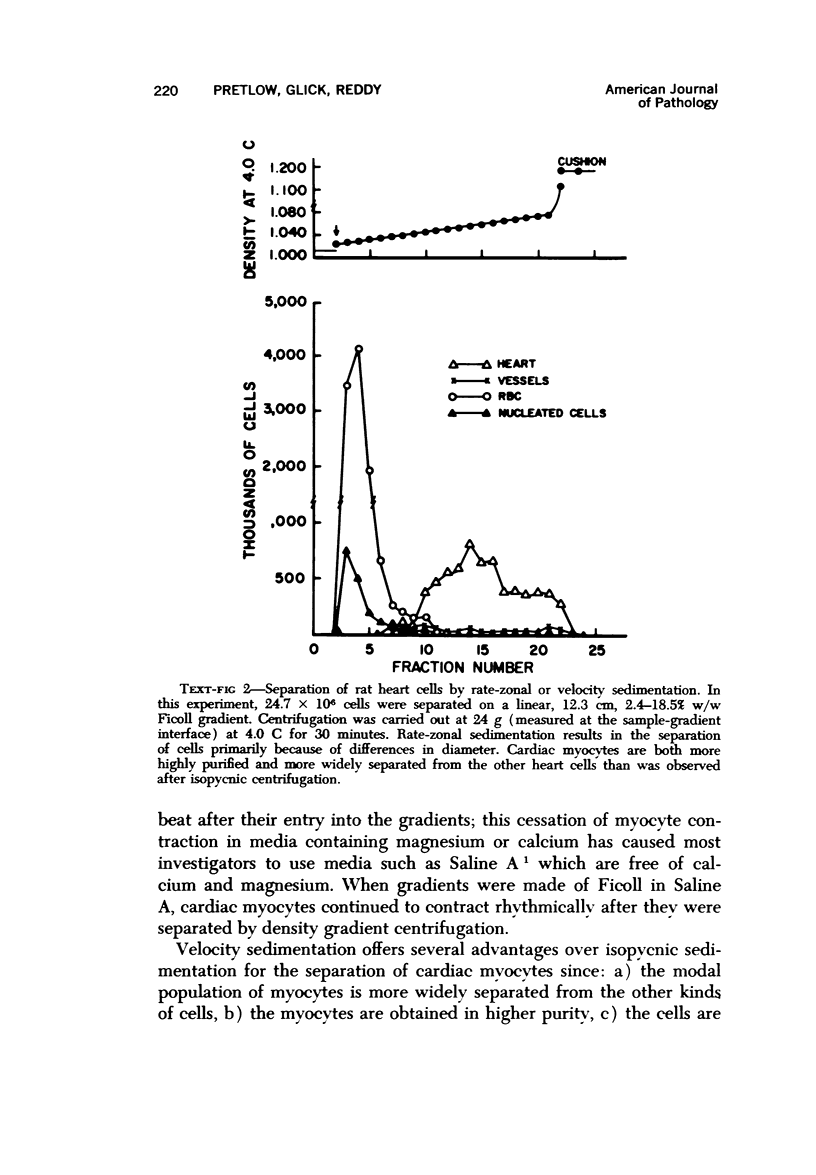
Heart cells were obtained in suspension after incubation with collagenase and hyaluronidase in Saline A. Cardiac myocytes were separated by isopycnic centrifugation in 88.6 to 92.4% purity from other heart cells with different densities, and by velocity or rate-zonal sedimentation, in 92.8 to 97.4% purity from heart cells with different diameters. A previously described computer integration of the differential sedimentation equation was used to determine the centrifugal force, duration of centrifugation and gradient design, which would permit the separation of cardiac myocytes from other heart cells by velocity sedimentation. The myocytes continued to contract rhythmically after being recovered from the density gradients. Velocity sedimentation was superior to isopycnic sedimentation for the separation of cardiac myocytes from heart cell suspensions because it gave the most highly purified myocytes, resulted in recovery of the largest proportion of myocytes in purified fractions from the gradient and required lower centrifugal forces for shorter periods of time. The potential significance of the availability of pure cardiac myocytes is discsused.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeloff M. D., Mangi R. J., Pretlow T. G., Mardiney M. R., Jr Isolation of leukemic blasts from peripheral blood by density gradient centrifugation. J Lab Clin Med. 1970 Apr;75(4):703–710. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S., Scheuer J. Morphology and metabolism of intact muscle cells isolated from adult rat heart. Circ Res. 1970 Jun;26(6):679–687. doi: 10.1161/01.res.26.6.679. [DOI] [PubMed] [Google Scholar]
- Bloom S. Spontaneous rhythmic contraction of separated heart muscle cells. Science. 1970 Mar 27;167(3926):1727–1729. doi: 10.1126/science.167.3926.1727. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Harell G. S., Bond H. E. The resolution of mixtures of viable mammalian cells into homogeneous fractions by zonal centrifugation. J Cell Biol. 1968 Feb;36(2):369–378. doi: 10.1083/jcb.36.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. M., Jr Carbohydrate metabolism in the isolated fetal rat heart. Am J Physiol. 1971 Mar;220(3):583–588. doi: 10.1152/ajplegacy.1971.220.3.583. [DOI] [PubMed] [Google Scholar]
- DeHann R. L. Regulation of spontaneous activity and growth of embryonic chick heart cells in tissue culture. Dev Biol. 1967 Sep;16(3):216–249. doi: 10.1016/0012-1606(67)90025-5. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Halbert S. P., Bruderer R., Lin T. M. In vitro organization of dissociated rat cardiac cells into beating three-dimensional structures. J Exp Med. 1971 Apr 1;133(4):677–695. doi: 10.1084/jem.133.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle W., Wollenberger A. Differentiation and behavior of isolated embryonic and neonatal heart cells in a chemically defined medium. Am J Cardiol. 1970 Mar;25(3):292–299. doi: 10.1016/s0002-9149(70)80006-6. [DOI] [PubMed] [Google Scholar]
- Hilfer S. R., Brown J. M. Collagenase. Its effectiveness as a dispersing agent for embryonic chick thyroid and heart. Exp Cell Res. 1971 Mar;65(1):246–249. doi: 10.1016/s0014-4827(71)80074-5. [DOI] [PubMed] [Google Scholar]
- McCarl R. L., Margossian S. S. Restoration of beating and enzymatic response of cultured rat heart cells to cortisol acetate. Arch Biochem Biophys. 1969 Mar;130(1):321–325. doi: 10.1016/0003-9861(69)90039-3. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Centrifugation of mammalian cells on gradients: a new rotor. Science. 1968 Aug 30;161(3844):911–913. doi: 10.1126/science.161.3844.911. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of malignant cells from transplantable rodent tumors. Exp Mol Pathol. 1970 Jun;12(3):249–256. doi: 10.1016/0014-4800(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of mammalian cells using programmed gradient sedimentation. Exp Mol Pathol. 1969 Oct;11(2):139–152. doi: 10.1016/0014-4800(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W., Shrager R. I., Weiss G. H. Rate zonal centrifugation in a Ficoll gradient. Anal Biochem. 1969 May;29(2):230–237. doi: 10.1016/0003-2697(69)90306-6. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Cassady I. M. Separation of mast cells in successive stages of differentiation using programmed gradient sedimentation. Am J Pathol. 1970 Dec;61(3):323–340. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Pichichero M. E., Hyams L. Separation of lymphocytes and macrophages from suspensions of guinea pig peritonitis exudate cells using programmed gradient sedimentation. Am J Pathol. 1971 May;63(2):255–276. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., Pushparaj N. A new method for separating peritoneal lymphocytes from macrophages. Immunology. 1972 Jan;22(1):87–91. [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Vodopick N., Takeda S., Lee W. H., Faulcon F. M. Serine requirement in leukemic and normal blood cells. Science. 1969 Mar 28;163(3874):1452–1453. doi: 10.1126/science.163.3874.1452. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Oratz M., Evans C. D., Gueyikian I., Rothschild M. A. Myosin, myoglobin, and collagen synthesis in acute cardiac overload. Am J Physiol. 1970 Aug;219(2):481–486. doi: 10.1152/ajplegacy.1970.219.2.481. [DOI] [PubMed] [Google Scholar]
- Seraydarian M. W., Sato E., Harary I. In vitro studies of beatg heart cells in culture. 13. The effect of 1-fluoro-2,4-dinitrobenzene. Arch Biochem Biophys. 1970 May;138(1):233–238. doi: 10.1016/0003-9861(70)90303-6. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Wei R., Starkweather R., Davis C. Preparation of beating heart cells from adult rats. Science. 1970 Mar 20;167(3925):1616–1618. doi: 10.1126/science.167.3925.1616. [DOI] [PubMed] [Google Scholar]