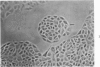
Abstract
Liver cultures offer several special advantages for the study of chemical carcinogenesis in cell culture; these include the sensitivity of the cells to procarcinogens requiring enzymatic activation, the epithelial nature of the cells which qualifies them as a model for epithelial carcinogenesis, and the opportunity to compare culture findings with the extensive information available on the effects of carcinogens on liver. The actions of chemical carcinogens have been studied in primary and long-term rat liver cell cultures. A variety of procarcinogens induced DNA repair in primary cultures, indicating the usefulness of this system for studying carcinogen metabolism, the interaction of carcinogens with DNA, and the repair of carcinogen-induced DNA damage. In addition, this system may provide a screen for chemical carcinogens in which metabolic activation occurs in the target cell. Carcinogen treatment of long-term cultures initiated from the primary cultures resulted in morphologic transformation accompanied by an increased growth in soft agar and an increased frequency of 8-azaguanine-resistant mutants. Cultures with a high fraction of cells in S phase were found to be most sensitive to the induction of 8-azaguanine-resistant mutants.
Full text
PDF

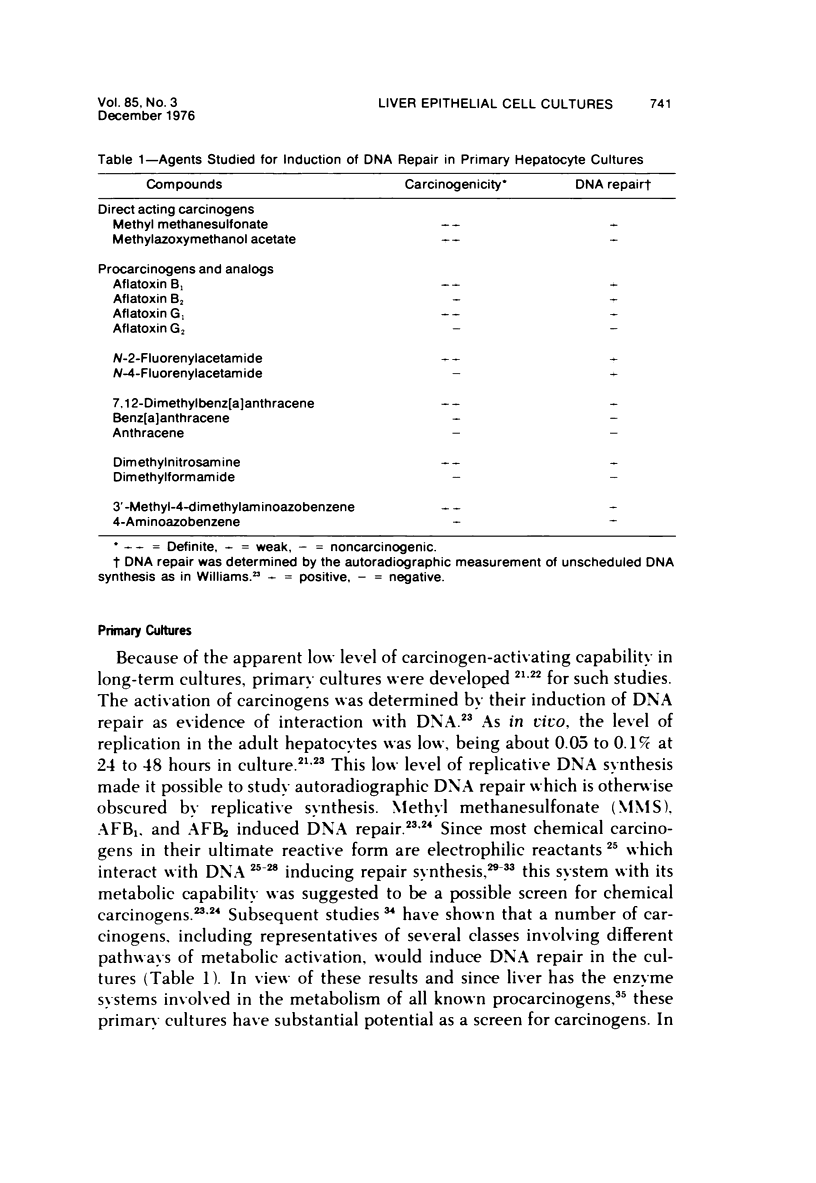



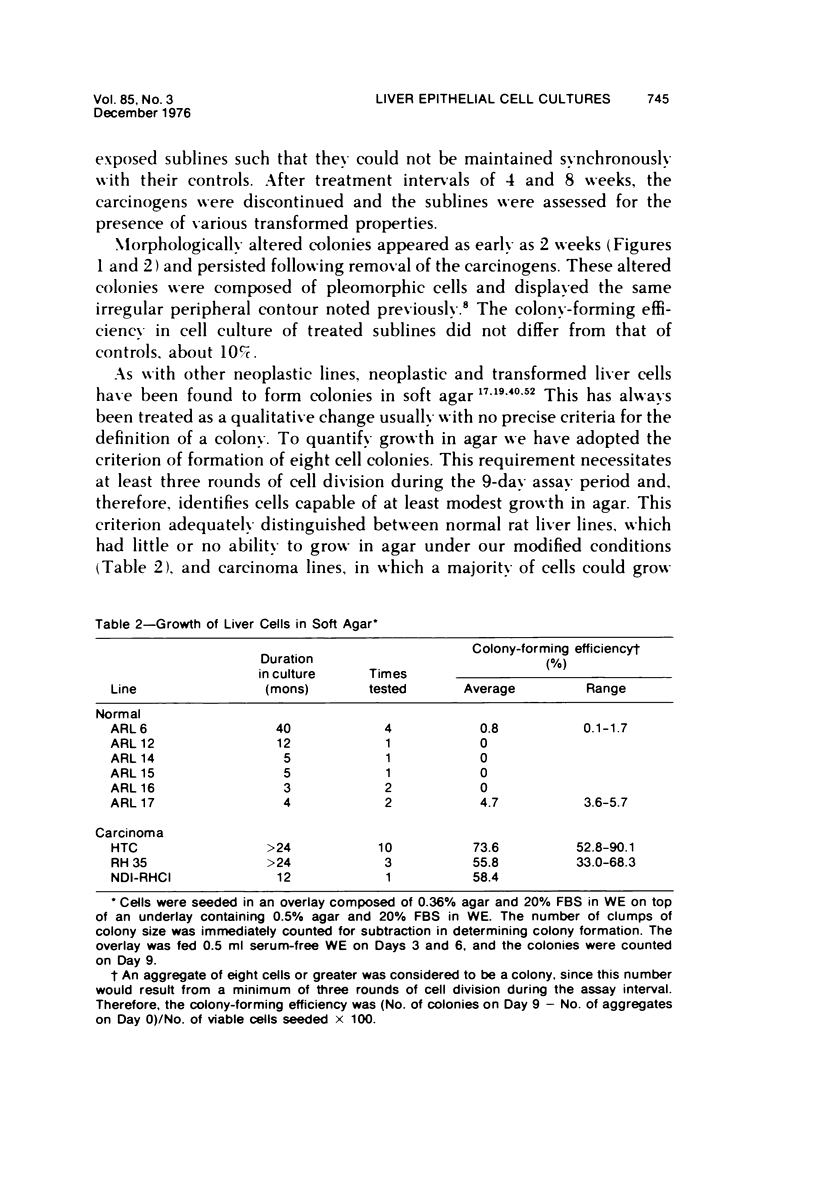
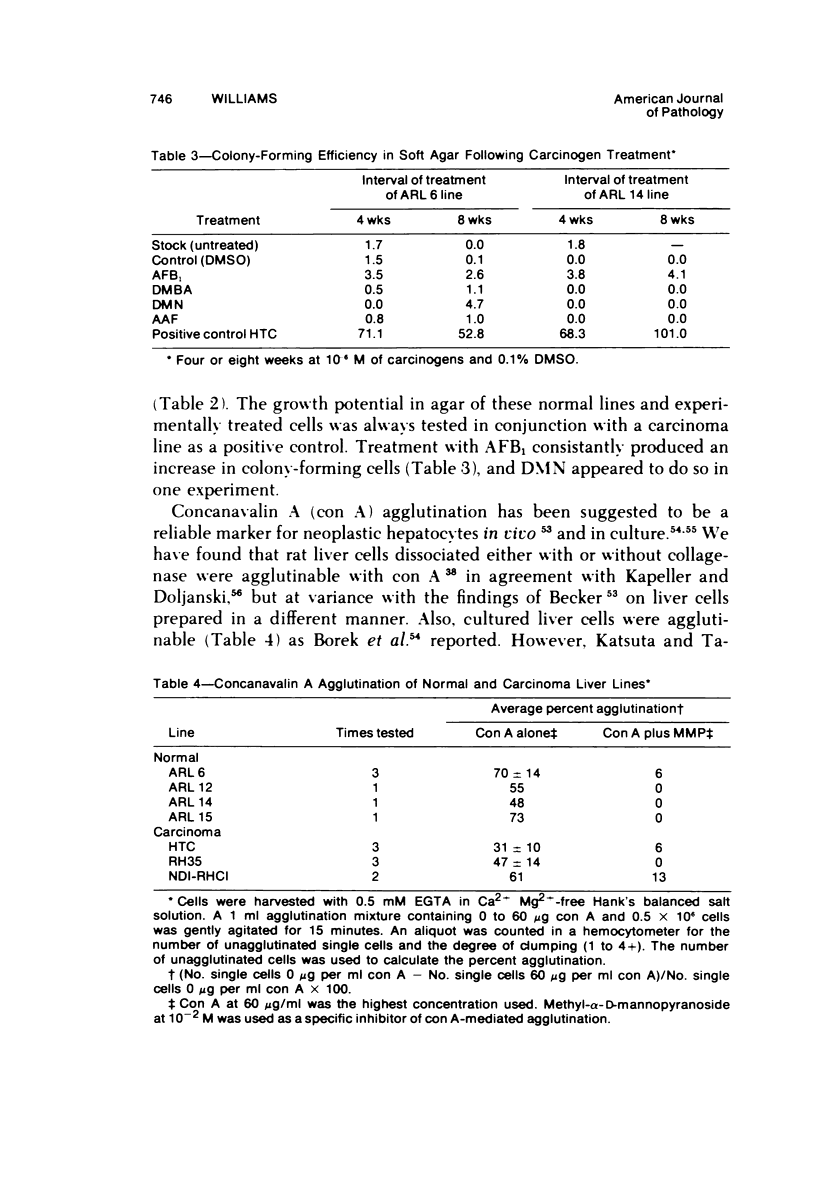




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERENBLUM I. A speculative review; the probable nature of promoting action and its significance in the understanding of the mechanism of carcinogenesis. Cancer Res. 1954 Aug;14(7):471–477. [PubMed] [Google Scholar]
- Bertram J. S., Heidelberger C. Cell cycle dependency of oncogenic transformation induced by N-methyl-N'-nitro-N-nitrosoquanidine in culture. Cancer Res. 1974 Mar;34(3):526–537. [PubMed] [Google Scholar]
- Berzins K., Blomberg F. Identification of concanavalin A-binding plasma membrane antigens of rat liver. FEBS Lett. 1975 Jun 15;54(2):139–143. doi: 10.1016/0014-5793(75)80061-5. [DOI] [PubMed] [Google Scholar]
- Borek C. Neoplastic transformation in vitro of a clone of adult liver epithelial cells into differentiated hepatoma-like cells under conditions of nutritional stress. Proc Natl Acad Sci U S A. 1972 Apr;69(4):956–959. doi: 10.1073/pnas.69.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E., Higgins P. J., Steinglass M., Bendich A. Properties and malignant transformation of established rat liver parenchymal cells in culture. J Natl Cancer Inst. 1975 Aug;55(2):375–384. [PubMed] [Google Scholar]
- Borenfreund E., Krim M., Sanders F. K., Sternberg S. S., Bendich A. Malignant conversion of cells in vitro by carcinogens and viruses. Proc Natl Acad Sci U S A. 1966 Aug;56(2):672–679. doi: 10.1073/pnas.56.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P. On the interaction of carcinogens with DNA. Biochem Pharmacol. 1971 May;20(5):999–1003. doi: 10.1016/0006-2952(71)90324-8. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Liver carcinomas induced in rats by single administration of dimethylnitrosamine after partial hepatectomy. J Natl Cancer Inst. 1971 Oct;47(4):899–907. [PubMed] [Google Scholar]
- Diamond L., McFall R., Tashiro Y., Sabatini D. The WIRL-3 rat liver cell lines and their transformed derivatives. Cancer Res. 1973 Nov;33(11):2627–2636. [PubMed] [Google Scholar]
- Dipaolo J. A., Donovan P. J. Properties of Syrian hamster cells transformed in the presence of carcinogenic hydrocarbons. Exp Cell Res. 1967 Nov;48(2):361–377. doi: 10.1016/0014-4827(67)90361-8. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Harsono T. Increased susceptibility to low doses of a carcinogen of epidermal cells in stimulated DNA synthesis. Cancer Res. 1967 Aug;27(8):1482–1484. [PubMed] [Google Scholar]
- Glimelius B., Nilsson K., Pontén J. Lectin agglutinability of non-neoplastic and neoplastic human lymphoid cells in vitro. Int J Cancer. 1975 Jun 15;15(6):888–896. doi: 10.1002/ijc.2910150604. [DOI] [PubMed] [Google Scholar]
- Heidelberger C., Iype P. T. Malignant transformation in vitro by carcinogenic hydrocarbons. Science. 1967 Jan 13;155(3759):214–217. [PubMed] [Google Scholar]
- Katsuta H., Takaoka T. Carcinogenesis in tissue culture. XIV. Malignant transformation of rat liver parenchymal cells treated with 4-nitroquinoline 1-oxide in tissue culture. J Natl Cancer Inst. 1972 Dec;49(6):1563–1576. doi: 10.1093/jnci/49.6.1563. [DOI] [PubMed] [Google Scholar]
- Koshiba K., Namba M., Oda T. Electron microscopic studies on cultured rat liver cells transformed by 4-nitroquinoline 1-oxide. Gan. 1970 Jun;61(3):233–238. [PubMed] [Google Scholar]
- Laishes B. A., Stich H. F. Repair synthesis and sedimentation analysis of DNA of human cells exposed to dimethylnitrosamine and activated dimethylnitrosamine. Biochem Biophys Res Commun. 1973 Jun 8;52(3):827–833. doi: 10.1016/0006-291x(73)91012-7. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- Marquardt H. Cell cycle dependence of chemically induced malignant transformation in vitro. Cancer Res. 1974 Jul;34(7):1612–1615. [PubMed] [Google Scholar]
- Marquardt H., Sternberg S. S., Philips F. S. 7,12-dimethylbenz(a)anthracene and hepatic neoplasia in regenerating rat liver. Chem Biol Interact. 1970 Dec;2(4):401–403. doi: 10.1016/0009-2797(70)90060-8. [DOI] [PubMed] [Google Scholar]
- Michael R. O., Williams G. M. Chloroquine inhibition of repair of DNA damage induced in mammalian cells by methyl methanesulfonate. Mutat Res. 1974 Dec;25(3):391–396. doi: 10.1016/0027-5107(74)90068-2. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Drevon C., Tomatis L. Production of epithelial and mesenchymal tumours with rat liver cells transformed in vitro. Int J Cancer. 1975 Oct 15;16(4):550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Tomatis L. Malignant transformation in vitro of rat liver cells by dimethylnitrosamine and N-methyl-N'-nitro-N-nitrosoguanidine. Br J Cancer. 1973 Sep;28(3):215–220. doi: 10.1038/bjc.1973.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba M., Masuji H., Sato J. Carcinogenesis in tissue culture. IX. Malignant transformation of cultured rat cells treated with 4-nitroquinoline-1-oxide. Jpn J Exp Med. 1969 Jun;39(3):253–265. [PubMed] [Google Scholar]
- Oshiro Y., Gerschenson L. E., DiPaolo J. A. Carcinomas from rat liver cells transformed spontaneously in culture. Cancer Res. 1972 Apr;32(4):877–879. [PubMed] [Google Scholar]
- Owens I. S., Nebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver-derived cell cultures. Stimulation of "cytochrome P1-450-associated" enzyme activity by many inducing compounds. Mol Pharmacol. 1975 Jan;11(1):94–104. [PubMed] [Google Scholar]
- Pound A. W. Carcinogenesis and cell proliferation. N Z Med J. 1968 Jan;67(426 Suppl):88–99. [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M., Plant J. E., Sturrock J. E., Crathorn A. R. Quantitative aspects of the repair of alkylated DNA in cultured mammalian cells. I. The effect on HeLa and Chinese hamster cell survival of alkylation of cellular macromolecules. Chem Biol Interact. 1971 Feb;3(1):29–47. doi: 10.1016/0009-2797(71)90024-x. [DOI] [PubMed] [Google Scholar]
- Roth J., Neupert G., Bolck F. Concanavalin A receptors in the plasma membrane of rat liver cells: comparative electron microscopic studies on normal cells and on cells in vivo transformed by diethylnitrosamine. Exp Pathol (Jena) 1975;10(3-4):143–155. doi: 10.1016/s0014-4908(75)80018-4. [DOI] [PubMed] [Google Scholar]
- San R. H., Stich H. F. DNA repair and chromatid anomalies in mammalian cells exposed to 4-nitroquinoline I-oxide. Mutat Res. 1970 Oct;10(4):389–404. doi: 10.1016/0027-5107(70)90051-5. [DOI] [PubMed] [Google Scholar]
- Sato J., Namba M., Usui K., Nagano D. Carcinogenesis in tissue culture. 8. Spontaneous malignant transformation of rat liver cells in long-term culture. Jpn J Exp Med. 1968 Apr;38(2):105–118. [PubMed] [Google Scholar]
- Sato J., Yabe T. Carcinogenesis in tissue culture. V. Effects of long-term addition of 4-dimethyl-aminoazobenzene and 3'-methyl-4-dimethyl-aminoazobenzene on liver cells in culture. Jpn J Exp Med. 1965 Oct;35(5):445–462. [PubMed] [Google Scholar]
- Schwartz A. G. Protective effect of benzoflavone and estrogen against 7,12-dimethylbenz(a)anthracene- and aflatoxin-induced cytotoxicity in cultured liver cells. Cancer Res. 1974 Jan;34(1):10–15. [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D. Defective repair of N-acetoxy-2-acetylaminofluorene-induced lesions in the DNA of xeroderma pigmentosum cells. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1019–1024. doi: 10.1016/s0006-291x(72)80243-2. [DOI] [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Studies with carcinogens and tumor-promoting agents in cell culture. Exp Cell Res. 1968 Mar;49(3):572–583. doi: 10.1016/0014-4827(68)90205-x. [DOI] [PubMed] [Google Scholar]
- Sullman S. F., Armstrong S. J., Zuckerman A. J., Rees K. R. Further studies on the toxicity of the aflatoxins on human cell cultures. Br J Exp Pathol. 1970 Jun;51(3):314–316. [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Hiasa Y., Ito N., Tsubura Y. In vitro malignant transformation of cells derived from rat liver by means of aflatoxin B1. Gan. 1970 Dec;61(6):557–561. [PubMed] [Google Scholar]
- Umeda M., Saito M. Carcinogenesis in tissue culture. XI. Cytotoxic effects of 4-dimethylaminoazobenzene and its derivatives on HeLa cells and on rat liver and kidney cells in culture. Jpn J Exp Med. 1969 Dec;39(6):601–613. [PubMed] [Google Scholar]
- Warwick G. P. Effect of the cell cycle on carcinogenesis. Fed Proc. 1971 Nov-Dec;30(6):1760–1765. [PubMed] [Google Scholar]
- Weinstein I. B., Orenstein J. M., Gebert R., Kaighn M. E., Stadler U. C. Growth and structural properties of epithelial cell cultures established from normal rat liver and chemically induced hepatomas. Cancer Res. 1975 Jan;35(1):253–263. [PubMed] [Google Scholar]
- Weinstein I. B., Yamaguchi N., Gebert R., Kaighn M. E. Use of epithelial cell cultures for studies on the mechanism of transformation by chemical carcinogens. In Vitro. 1975 May-Jun;11(3):130–141. doi: 10.1007/BF02615421. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Carcinogen-induced DNA repair in primary rat liver cell cultures; a possible screen for chemical carcinogens. Cancer Lett. 1976 Mar;1(4):231–235. doi: 10.1016/s0304-3835(75)97171-2. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Elliott J. M., Weisburger J. H. Carcinoma after malignant conversion in vitro of epithelial-like cells from rat liver following exposure to chemical carcinogens. Cancer Res. 1973 Mar;33(3):606–612. [PubMed] [Google Scholar]
- Williams G. M. Functional markers and growth behavior of preneoplastic hepatocytes. Cancer Res. 1976 Jul;36(7 Pt 2):2540–2543. [PubMed] [Google Scholar]
- Williams G. M., Gunn J. M. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974 Nov;89(1):139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Primary and long-term culture of adult rat liver epithelial cells. Methods Cell Biol. 1976;14:357–364. doi: 10.1016/s0091-679x(08)60495-1. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Stromberg K., Krodes R. Cytochemical and ultrastructural alterations associated with confluent growth in cell cultures of epithelial-like cells from rat liver. Lab Invest. 1973 Sep;29(3):293–303. [PubMed] [Google Scholar]
- Williams G. M., Weisburger E. K., Weisburger J. H. Isolation and long-term cell culture of epithelial-like cells from rat liver. Exp Cell Res. 1971 Nov;69(1):106–112. doi: 10.1016/0014-4827(71)90316-8. [DOI] [PubMed] [Google Scholar]