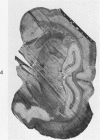
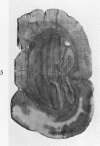
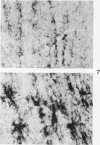
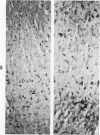
Full text
PDF













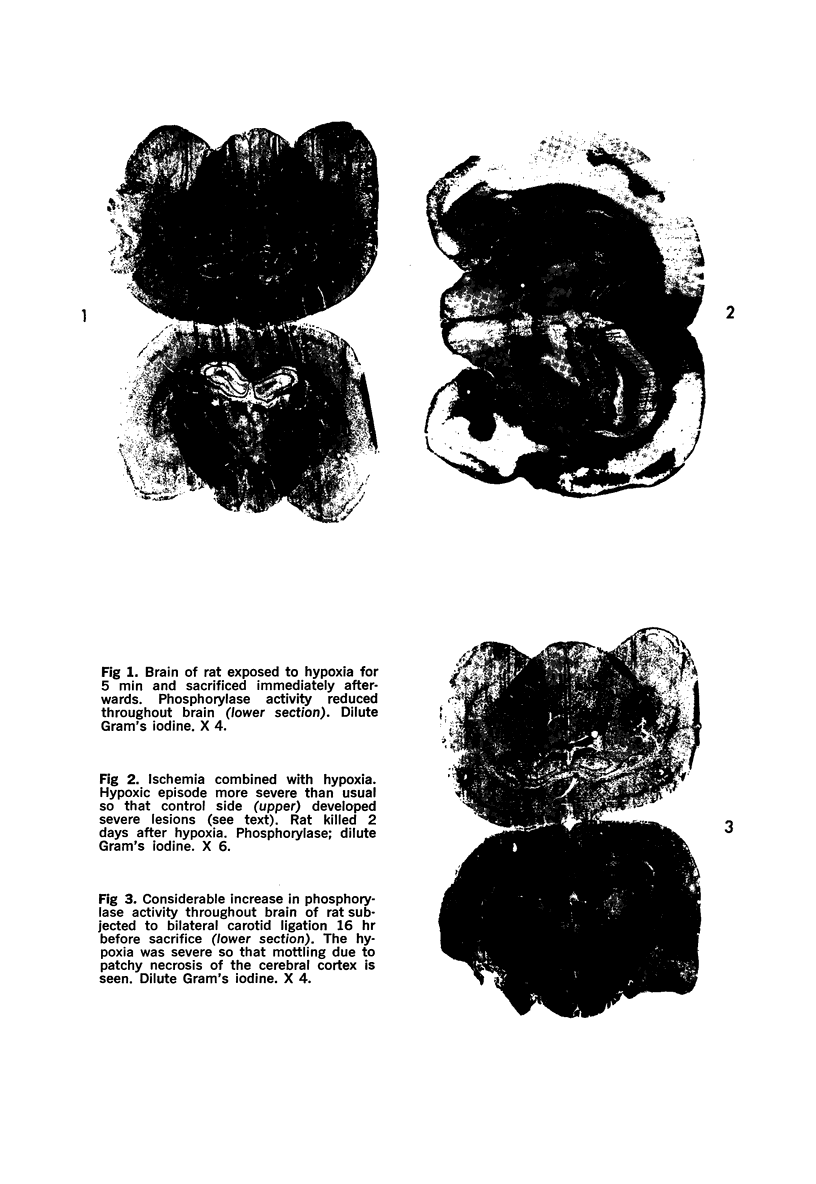
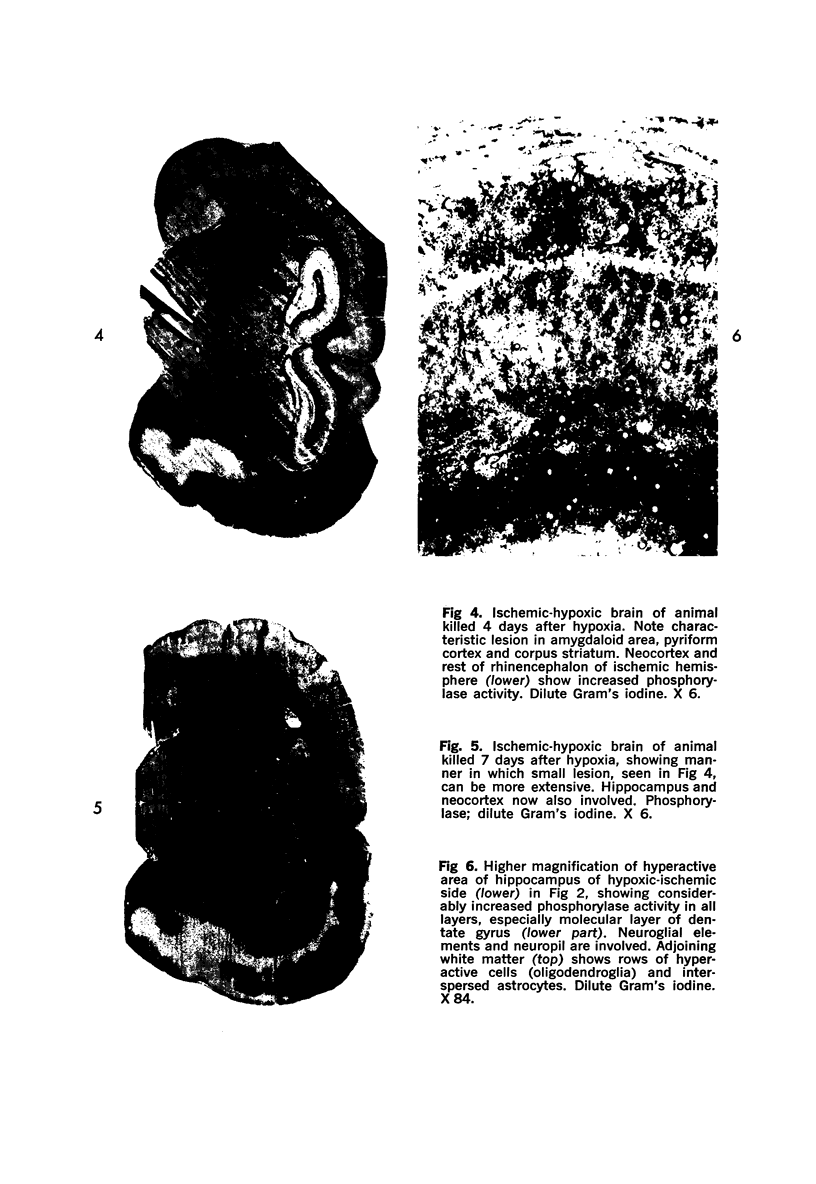

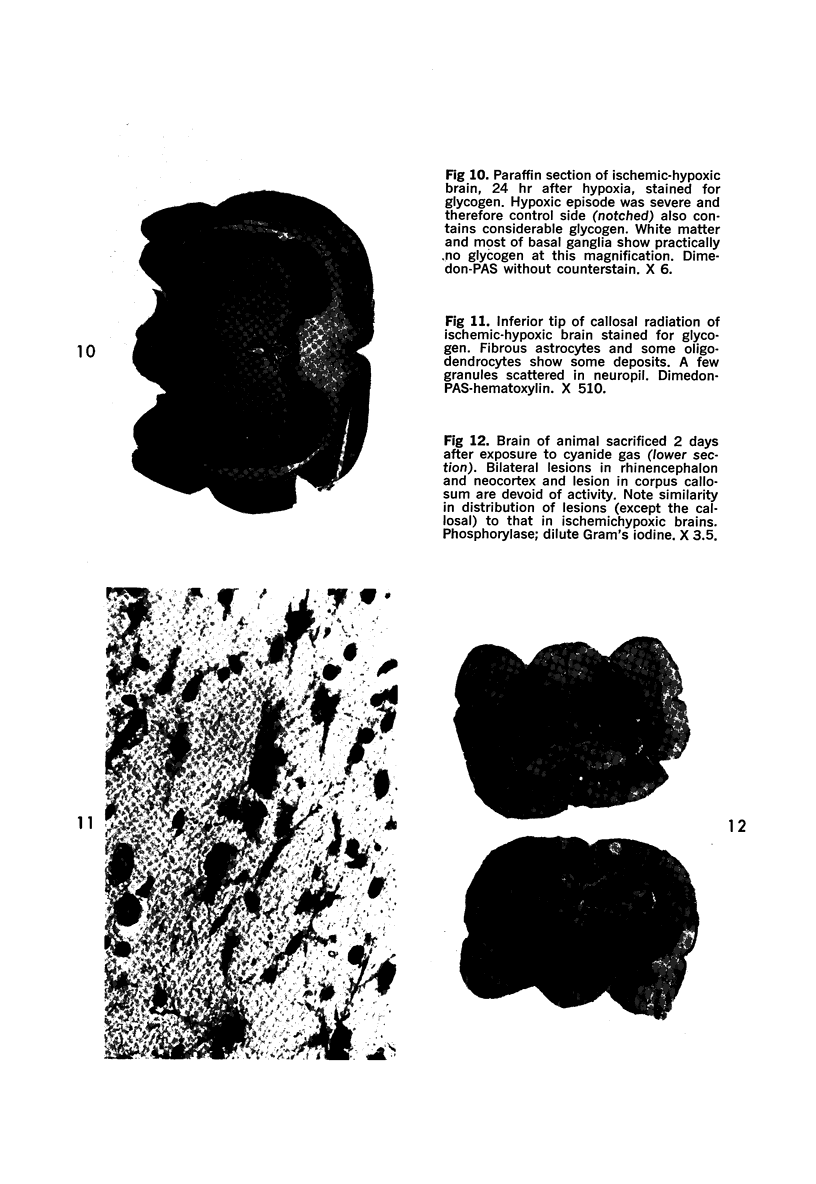
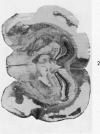
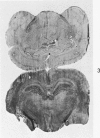
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBAUM H. G., CHINN H. I. Brain metabolism during acclimatization to high altitude. Am J Physiol. 1953 Jul;174(1):141–145. doi: 10.1152/ajplegacy.1953.174.1.141. [DOI] [PubMed] [Google Scholar]
- BECKER N. H., BARRON K. D. The cytochemistry of anoxic and anoxic-ischemic encephalopathy in rats. I. Alterations in neuronal lysosomes identified by acid phosphatase activity. Am J Pathol. 1961 Feb;38:161–175. [PMC free article] [PubMed] [Google Scholar]
- BECKER N. H. The cytochemistry of anoxic and anoxioischemic encephalopathy in rats. II. Alterations in neuronal mitochondria indentified by diphosphopyridine and triphosphopyridine nucleotide diaphorases. Am J Pathol. 1961 May;38:587–597. [PMC free article] [PubMed] [Google Scholar]
- BRECKENRIDGE B. M., NORMAN J. H. Glycogen phosphorylase in brain. J Neurochem. 1962 Jul-Aug;9:383–392. doi: 10.1111/j.1471-4159.1962.tb09464.x. [DOI] [PubMed] [Google Scholar]
- BULMER D. Dimedone as an aldehyde blocking reagent to facilitate the histochemical demonstration of glycogen. Stain Technol. 1959 Mar;34(2):95–98. doi: 10.3109/10520295909114656. [DOI] [PubMed] [Google Scholar]
- Bakay L., Lee J. C. The effect of acute hypoxia and hypercapnia on the ultrastructure of the central nervous system. Brain. 1968;91(4):697–706. doi: 10.1093/brain/91.4.697. [DOI] [PubMed] [Google Scholar]
- De Piras M. M., Zadunaisky J. A. Effect of potassium and oubain on glucose metabolism by frog brain. J Neurochem. 1965 Jul;12(7):657–661. doi: 10.1111/j.1471-4159.1965.tb04259.x. [DOI] [PubMed] [Google Scholar]
- ERANKO O., PALKAMA A. Improved localization of phosphorylase by the use of polyvinyl pyrrolidone and high substrate concentration. J Histochem Cytochem. 1961 Sep;9:585–585. doi: 10.1177/9.5.585. [DOI] [PubMed] [Google Scholar]
- FRIEDE R. L. Histochemical distribution of phosphorylase in the brain of the guinea-pig. J Neurol Neurosurg Psychiatry. 1959 Nov;22:325–329. doi: 10.1136/jnnp.22.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDE R. Uber die trophische Funktion der Glia. Virchows Arch Pathol Anat Physiol Klin Med. 1953;324(1):15–26. doi: 10.1007/BF00948092. [DOI] [PubMed] [Google Scholar]
- Gatfield P. D., Lowry O. H., Schulz D. W., Passonneau J. V. Regional energy reserves in mouse brain and changes with ischaemia and anaesthesia. J Neurochem. 1966 Mar;13(3):185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- HICKS S. P. Brain metabolism in vivo; the distribution of lesions caused by cyanide poisoning, insulin hypoglycemia, asphyxia in nitrogen and fluoroacetate poisoning in rats. AMA Arch Pathol. 1950 Feb;49(2):111-37, illust. [PubMed] [Google Scholar]
- HIMMELHOCH S. R., KARNOVSKY M. J. The histochemical demonstration of glyceraldehyde-3-phosphate dehydrogenase activity. J Biophys Biochem Cytol. 1961 Mar;9:573–581. doi: 10.1083/jcb.9.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORNBROOK K. R., BRODY T. M. The effect of catecholamines on muscle glycogen and phosphorylase activity. J Pharmacol Exp Ther. 1963 Jun;140:295–307. [PubMed] [Google Scholar]
- Hirano A., Levine S., Zimmerman H. M. Experimental cyanide encephalopathy: electron microscopic observations of early lesions in white matter. J Neuropathol Exp Neurol. 1967 Apr;26(2):200–213. doi: 10.1097/00005072-196704000-00002. [DOI] [PubMed] [Google Scholar]
- IBRAHIM M. Z., BRISCOE P. B., Jr, BAYLISS O. B., ADAMS C. W. THE RELATIONSHIP BETWEEN ENZYME ACTIVITY AND NEUROGLIA IN THE PRODROMAL AND DEMYELINATING STAGES OF CYANIDE ENCEPHALOPATHY IN THE RAT. J Neurol Neurosurg Psychiatry. 1963 Dec;26:479–486. doi: 10.1136/jnnp.26.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. Z., Adams C. W. The relation between enzyme activity and neuroglia in early plaques of multiple sclerosis. J Pathol Bacteriol. 1965 Jul;90(1):239–243. doi: 10.1002/path.1700900126. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. Z., Castellani P. Demonstration of phosphorylase activity in the rat brain. Histochemie. 1968;16(1):9–14. doi: 10.1007/BF00306207. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. Z., Levine S. Effect of cyanide intoxication on the metachromatic material found in the central nervous system. J Neurol Neurosurg Psychiatry. 1967 Dec;30(6):545–555. doi: 10.1136/jnnp.30.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. Z., Miquel J., Haymaker W. Glycogen, phosphorylase and branching enzyme in experimental pathological conditions of the rat brain. J Neuropathol Exp Neurol. 1968 Jan;27(1):119–121. [PubMed] [Google Scholar]
- Kay R. E., Chan H. Effect of x-irradiation on glucose metabolism in rat cerebral cortex slices. J Neurochem. 1967 Apr;14(4):401–403. doi: 10.1111/j.1471-4159.1967.tb09537.x. [DOI] [PubMed] [Google Scholar]
- LEVINE S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960 Jan;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., KLEIN M. Ischemic infarction and swelling in the rat brain. Arch Pathol. 1960 May;69:544–553. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LUCAS B. G., STRANGEWAYS D. H. EXPERIMENTAL CEREBRAL ANOXIA. J Pathol Bacteriol. 1963 Oct;86:273–281. doi: 10.1002/path.1700860202. [DOI] [PubMed] [Google Scholar]
- Maker H. S., Lehrer G. M., Weiss C., Silides D. J., Scheinberg L. C. The quantitative histochemistry of a chemically induced ependymoblastoma. II. The effect of ischaemia on substrates of carbohydrate metabolism. J Neurochem. 1966 Nov;13(11):1207–1212. doi: 10.1111/j.1471-4159.1966.tb04278.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. R., Schulz D. W., Passonneau J. V., Lowry O. H. Control of glycogen levels in brain. J Neurochem. 1968 Nov;15(11):1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- PARMEGGIANI A., MORGAN H. E. Effect of adenine nucleotides and inorganic phosphate on muscle phosphorylase activity. Biochem Biophys Res Commun. 1962 Oct 17;9:252–256. doi: 10.1016/0006-291x(62)90068-2. [DOI] [PubMed] [Google Scholar]
- PASSONNEAU J. V., LOWRY O. H. Phosphofructokinase and the Pasteur effect. Biochem Biophys Res Commun. 1962 Feb 20;7:10–15. doi: 10.1016/0006-291x(62)90134-1. [DOI] [PubMed] [Google Scholar]
- Prasannan K. G., Subrahmanyam K. Enzymes of glycogen metabolism in cerebral cortex of normal and diabetic rats. J Neurochem. 1968 Oct;15(10):1239–1241. doi: 10.1111/j.1471-4159.1968.tb06843.x. [DOI] [PubMed] [Google Scholar]
- SELINGER Z., SCHRAMM M. An insoluble complex formed by the interaction of muscle phosphorylase with glycogen. Biochem Biophys Res Commun. 1963 Jul 26;12:208–214. doi: 10.1016/0006-291x(63)90191-8. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N., HAMURO Y. Deposition of glycogen and changes in some enzymes in brain wounds. Nature. 1958 Mar 15;181(4611):781–782. doi: 10.1038/181781a0. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N., KUMAMOTO T. Histochemical studies on the glycogen of the mammalian brain. Anat Rec. 1952 Nov;114(3):479–497. doi: 10.1002/ar.1091140307. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N., OKADA M. Histochemical distribution of phosphorylase in rodent brain from newborn to adults. J Histochem Cytochem. 1957 Sep;5(5):459–471. doi: 10.1177/5.5.459. [DOI] [PubMed] [Google Scholar]
- SPECTOR R. G. Water content of the brain in anoxic-ischaemic encephalopathy in adult rats. Br J Exp Pathol. 1961 Dec;42:623–630. [PMC free article] [PubMed] [Google Scholar]
- Schuberth J., Sollenberg J., Sundwall A., Sörbo B. Acetylcoenzyme A in brain. The effect of centrally active drugs, insulin coma and hypoxia. J Neurochem. 1966 Sep;13(9):819–822. doi: 10.1111/j.1471-4159.1966.tb05877.x. [DOI] [PubMed] [Google Scholar]
- Sie H. G., Sawyer D., Fishman W. H. Enzymorphologic demonstration of glucose 6-phosphate--dependent glycogen synthetase in mouse liver. J Histochem Cytochem. 1966 Mar;14(3):247–253. doi: 10.1177/14.3.247. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Moonsammy G. I. Substrate changes in peripheral nerve recovering from anoxia. J Neurochem. 1966 Dec;13(12):1433–1439. doi: 10.1111/j.1471-4159.1966.tb04304.x. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Passonneau J. V., Lowry O. H. Substrate changes in peripheral nerve during ischaemia and Wallerian degeneration. J Neurochem. 1965 Aug;12(8):719–727. doi: 10.1111/j.1471-4159.1965.tb06786.x. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T., GLENNER G. G. Histochemical demonstration of uridine diphosphate glucose-glycogen transferase in animal tissues. J Histochem Cytochem. 1961 May;9:304–316. doi: 10.1177/9.3.304. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T. Histochemical demonstration of branching enzyme (amylo-1,4-1,6-transglucosidase) in animal tissues. J Histochem Cytochem. 1958 May;6(3):208–216. doi: 10.1177/6.3.208. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T., KURIAKI H. Histochemical detection of phosphorylase in animal tissues. J Histochem Cytochem. 1955 May;3(3):153–160. doi: 10.1177/3.3.153. [DOI] [PubMed] [Google Scholar]
- THORN W., ISSELHARD W., MUELDENER B. [Glycogen, glucose and lactic content in warm-blooded animal organs in various experiments and anoxic stress ascertained with the aid of optical enzyme tests]. Biochem Z. 1959;331:545–562. [PubMed] [Google Scholar]
- VAN HOUTEN W. H., FRIEDE R. L. Histochemical studies of experimental demyelination produced with cyanide. Exp Neurol. 1961 Nov;4:402–412. doi: 10.1016/0014-4886(61)90026-7. [DOI] [PubMed] [Google Scholar]
- WEGMANN R., GERZELI G. [Glucose-6-phosphate dehydrogenase and its correlations with the similar substrates of glucose-6-phosphate. Role of hexokinase]. Ann Histochim. 1961 Apr-Jun;6:111–124. [PubMed] [Google Scholar]