Abstract
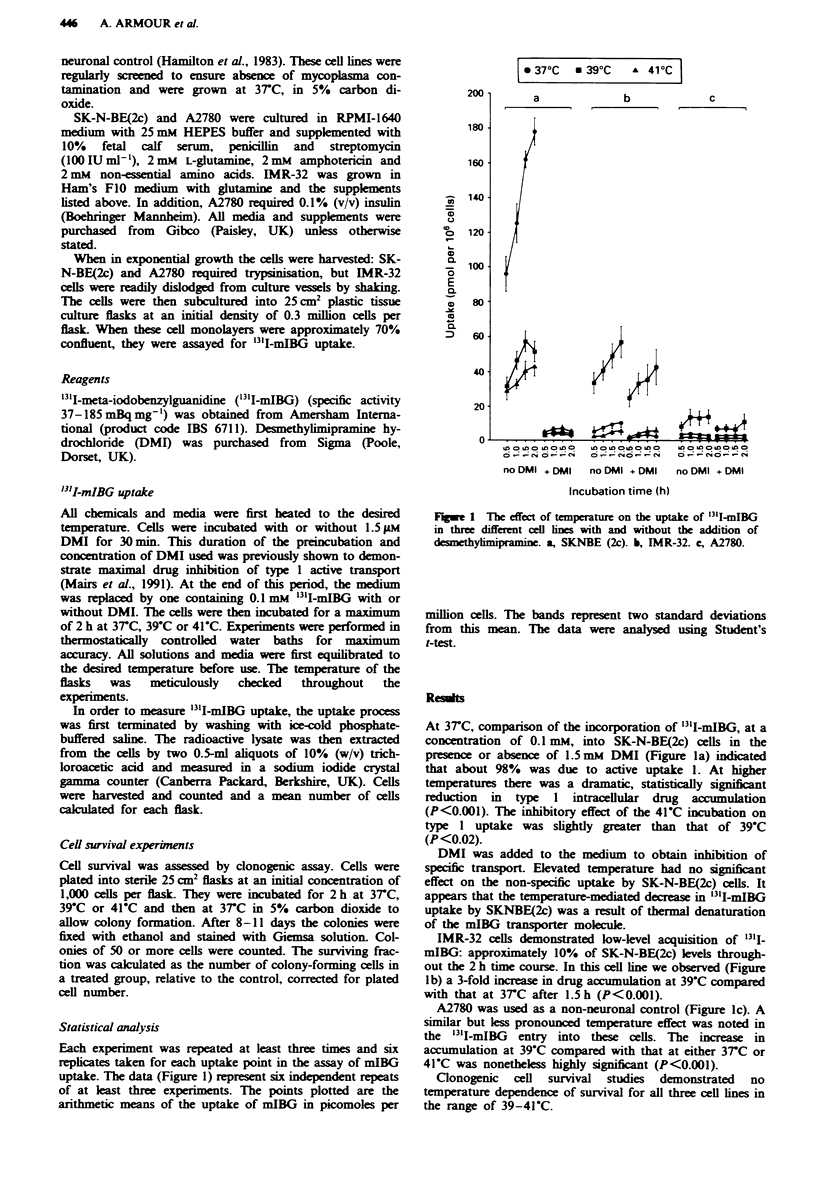
Successful imaging or treatment of neuroblastoma with 131I-meta-iodobenzylguanidine (131I-mIBG) depends on the selectivity of active (type 1) uptake of mIBG in neuroblastoma cells relative to passive (type 2) uptake present in most normal tissues. This study investigates the effects of moderately elevated temperature (39-41 degrees C) on the cellular uptake of 131I-mIBG in two neuroblastoma cell lines [SK-N-BE(2c) and IMR-32] and in a non-neuronal (ovarian carcinoma) cell line (A2780). In SK-N-BE(2c), a cell line with high active uptake capacity, the specific (type 1) uptake was reduced by 75% (P < 0.001) at 39 degrees C. Both IMR-32 and A2780 have a low capacity for accumulation of mIBG by active uptake. These cell lines demonstrated a statistically significant increase in accumulation at 39 degrees C, mainly as a result of increased non-specific transport. At 41 degrees C uptake of 131I-mIBG was reduced in all cell lines. Thus, the active component of mIBG uptake is more vulnerable to increased temperature than the passive component. It seems probable that moderately increased temperature will have an unfavourable effect on the therapeutic differential for targeted radiotherapy of neuroblastoma using radiolabelled mIBG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Elkind M. M., Bronk B. V. Thermally enhanced radioresponse of cultured Chinese hamster cells: inhibition of repair of sublethal damage and enhancement of lethal damage. Radiat Res. 1974 Apr;58(1):38–51. [PubMed] [Google Scholar]
- Buck J., Bruchelt G., Girgert R., Treuner J., Niethammer D. Specific uptake of m-[125I]iodobenzylguanidine in the human neuroblastoma cell line SK-N-SH. Cancer Res. 1985 Dec;45(12 Pt 1):6366–6370. [PubMed] [Google Scholar]
- Cheng K. H., Hui S. W., Lepock J. R. Protection of the membrane calcium adenosine triphosphatase by cholesterol from thermal inactivation. Cancer Res. 1987 Mar 1;47(5):1255–1262. [PubMed] [Google Scholar]
- Gerweck L. E., Gillette E. L., Dewey W. C. Effect of heat and radiation on synchronous Chinese hamster cells: killing and repair. Radiat Res. 1975 Dec;64(3):611–623. [PubMed] [Google Scholar]
- Gerweck L. E., Gillette E. L., Dewey W. C. Killing of Chinese hamster cells in vitro by heating under hypoxic or aerobic conditions. Eur J Cancer. 1974 Oct;10(10):691–693. doi: 10.1016/0014-2964(74)90009-7. [DOI] [PubMed] [Google Scholar]
- Hahn G. M. Metabolic aspects of the role of hyperthermia im mammalian cell inactivation and their possible relevance to cancer treatment. Cancer Res. 1974 Nov;34(11):3117–3123. [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Jaques S., Jr, Tobes M. C., Sisson J. C. Sodium dependency of uptake of norepinephrine and m-iodobenzylguanidine into cultured human pheochromocytoma cells: evidence for uptake-one. Cancer Res. 1987 Aug 1;47(15):3920–3928. [PubMed] [Google Scholar]
- Kwock L., Lin P. S., Hefter K., Wallach D. F. Impairment of Na+-dependent amino acid transport in a cultured human T-cell line by hyperthermia and irradiation. Cancer Res. 1978 Jan;38(1):83–87. [PubMed] [Google Scholar]
- Lampidis T. J., Castello C., del Giglio A., Pressman B. C., Viallet P., Trevorrow K. W., Valet G. K., Tapiero H., Savaraj N. Relevance of the chemical charge of rhodamine dyes to multiple drug resistance. Biochem Pharmacol. 1989 Dec 1;38(23):4267–4271. doi: 10.1016/0006-2952(89)90525-x. [DOI] [PubMed] [Google Scholar]
- Lashford L. S., Hancock J. P., Kemshead J. T. Meta-iodobenzylguanidine (mIBG) uptake and storage in the human neuroblastoma cell line SK-N-BE(2C). Int J Cancer. 1991 Jan 2;47(1):105–109. doi: 10.1002/ijc.2910470119. [DOI] [PubMed] [Google Scholar]
- Lepock J. R. Involvement of membranes in cellular responses to hyperthermia. Radiat Res. 1982 Dec;92(3):433–438. [PubMed] [Google Scholar]
- Mairs R. J., Gaze M. N., Barrett A. The uptake and retention of metaiodobenzyl guanidine by the neuroblastoma cell line NB1-G. Br J Cancer. 1991 Aug;64(2):293–295. doi: 10.1038/bjc.1991.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci L., Mondovì B. Biochemical and ultrastructural changes in the hyperthermic treatment of tumor cells: an outline. Adv Exp Med Biol. 1990;267:99–120. doi: 10.1007/978-1-4684-5766-7_9. [DOI] [PubMed] [Google Scholar]
- McLaren J. R., Pontiggia P. The basis for hyperthermia becoming the fourth cancer treatment modality in the 1990's. Adv Exp Med Biol. 1990;267:21–36. doi: 10.1007/978-1-4684-5766-7_2. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. Temperature sensitivity of the erythrocyte membrane potential as determined by cyanine dye fluorescence. Cell Biol Int Rep. 1977 Jan;1(1):51–55. doi: 10.1016/0309-1651(77)90009-1. [DOI] [PubMed] [Google Scholar]
- Moyes J. S., Babich J. W., Carter R., Meller S. T., Agrawal M., McElwain T. J. Quantitative study of radioiodinated metaiodobenzylguanidine uptake in children with neuroblastoma: correlation with tumor histopathology. J Nucl Med. 1989 Apr;30(4):474–480. [PubMed] [Google Scholar]
- Sapareto S. A., Hopwood L. E., Dewey W. C. Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res. 1978 Feb;73(2):221–233. [PubMed] [Google Scholar]
- Smets L. A., Janssen M., Metwally E., Loesberg C. Extragranular storage of the neuron blocking agent meta-iodobenzylguanidine (MIBG) in human neuroblastoma cells. Biochem Pharmacol. 1990 Jun 15;39(12):1959–1964. doi: 10.1016/0006-2952(90)90615-r. [DOI] [PubMed] [Google Scholar]
- Stiller C. A., Bunch K. J. Trends in survival for childhood cancer in Britain diagnosed 1971-85. Br J Cancer. 1990 Nov;62(5):806–815. doi: 10.1038/bjc.1990.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom R., Santoro A. S., Crifo' C., Bozzi A., Mondovi' B., Fanelli A. R. The biochemical mechanism of selective heat sensitivity of cancer cells. IV. Inhibition of RNA synthesis. Eur J Cancer. 1973 Feb;9(2):103–112. doi: 10.1016/0014-2964(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Suit H. D., Gerweck L. E. Potential for hyperthermia and radiation therapy. Cancer Res. 1979 Jun;39(6 Pt 2):2290–2298. [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Verma S. P., Schmidt-Ullrich R., Thompson W. S., Wallach D. F. Differences between the structural dynamics of plasma membranes of normal hamster lymphocytes and lymphoid cells neoplastically transformed by simian virus 40 as revealed by laser Raman spectroscopy. Cancer Res. 1977 Oct;37(10):3490–3493. [PubMed] [Google Scholar]
- Westra A., Dewey W. C. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(5):467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]


