Abstract
Kainate receptors mediate both direct excitatory and indirect modulatory actions in the CNS. We report here that kainate has both pre- and postsynaptic actions in layer II/III pyramidal neurons of rat prefrontal cortex. Application of low concentration of kainate (50-500 nM) increased the amplitude of evoked excitatory postsynaptic currents (EPSCs) whereas higher concentrations (3 μM) caused a decrease. The frequency of spontaneous and miniature (action potential-independent) EPSCs was increased by low concentrations of kainate without affecting their amplitudes, suggesting a presynaptic mechanism of action. The facilitatory and inhibitory effects of kainate were mimicked by the GluR5 subunit selective agonist ATPA. In addition to decreasing EPSC amplitudes, high concentrations of kainate and ATPA induced an inward current which was not blocked by AMPA- or NMDA- receptor antagonists GYKI52466 and D-APV, respectively. The inward currents were blocked by the AMPA/KA receptor antagonist CNQX, indicating the presence of postsynaptic kainate receptors. Single shock stimulation in the presence of GYKI52466 and D-APV evoked an EPSC which was blocked by CNQX. The GluR5 antagonist LY382884 changed paired-pulse facilitation to paired pulse depression, indicating that synaptically released glutamate can activate presynaptic kainate receptors. These results suggest that kainate receptors containing GluR5 subunits play a major role in glutamatergic transmission in rat neocortex, having both presynaptic modulatory and direct postsynaptic excitatory actions.
Keywords: neocortex, kainate receptors, EPSCs, modulation, GluR5
1. Introduction
Early studies comparing the distribution of mRNAs encoding for five different kainate receptor subunits (GluR5-7, KA-1, and KA-2) found that these subunits are all abundantly expressed in neocortex (Wisden and Seeburg, 1993). GluR5-7 are known to form functional homomeric glutamate receptors (Hollmann and Heinemann, 1994; Bettler and Mulle, 1995; Schiffer et al., 1997) whereas KA1 and KA2 do not (Werner et al., 1991; Herb et al., 1992). KA1 and KA2 can co-assemble with GluR5-7 to make channels with unique functional properties (Hollmann and Heinemann, 1994; Chittajallu et al., 1999). The existence of splice variants and mRNA editing suggests additional complexity in kainate receptor subunit composition (Chittajallu et al., 1999). The exact subunit stoichiometry of native kainate receptors in neocortex, or elsewhere, has not been determined. In addition, the role of individual subunits in kainate receptor-mediated responses in neocortex has not been established.
Advances in the study of kainate receptors and their role in synaptic transmission occurred when agents enabling pharmacological isolation of kainate-mediated responses became available (Paternain et al., 1995; Wilding and Huettner, 1995). Application of selective antagonists of N-methyl-D-aspartate (NMDA) (e.g., D-APV) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropioinic acid (AMPA) (e.g., GYKI52466 or SYM 2206) receptors allowed identification of kainate receptor-mediated responses. Pharmacological tools also exist to delineate effects mediated by kainate receptors containing GluR5 subunits. (RS)-2-amino-3-(3-hydroxy-5-tert-butyl-isoxazol-4-yl) proprionic acid (ATPA), at low concentrations, is a selective GluR5 agonist (Clarke et al., 1997) and the GluR5 subunit is selectively blocked by (3S, 4aR, 6S, 8aR)-6-(4-carboxyphenyl)methyl-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid (LY382884) (Bortolotto et al., 1999).
EPSCs mediated by postsynaptic kainate receptors have been described in pyramidal and fast-spiking cells in layers II/III and V of rat motor cortex (Ali, 2003). Kainate EPSCs have also been observed in neonatal layer IV neurons where they exhibit very slow kinetics (Kidd and Isaac, 2001). Kainate receptors are located along the entire somatodendritic compartment of layer V pyramidal neurons, mediating synaptic and extrasynaptic responses (Eder et al., 2003). Presynaptic kainate receptors located on synaptic terminals of presynaptic fast-spiking interneurons decrease GABA release onto layer V pyramidal cells (Ali et al., 2001). Information about pre- and postsynaptic kainate receptors on layer II/III pyramidal cells and their subunit composition is lacking.
Ionotropic glutamate receptors have traditionally been assumed to be located postsynaptically whereas metabotropic glutamate receptors are found both pre- and postsynaptically. This view has recently been undergoing revision since ionotropic kainate receptors are known to be located presynaptically in several brain regions including amygdala (Li et al., 2001), cerebellum (Delaney and Jahr, 2002), hippocampus (Vignes et al., 1998; Schmitz et al., 2001) and substantia nigra pars compacta (Nakamura et al., 2003) where they can either facilitate or depress synaptic transmission. In developing neocortex, high frequency stimulation or application of kainate receptor agonists produces a significant EPSC depression via presynaptic kainate receptors (Kidd et al., 2002). Glutamate release from isolated cerebral cortex nerve terminals (synaptosomes), evoked by 4-aminopyridine, is enhanced by presynaptic kainate receptors (Perkinton and Sihra, 1999), indicating that facilitatory autoreceptors are also present in neocortex. In the present study, we have examined the role of pre- and postsynaptic kainate receptors in regulating excitation of layer II/III pyramidal cells in rat neocortex. Our results indicate that kainate receptors containing GluR5 subunits tonically facilitate glutamate release and underlie kainate mediated EPSCs.
2. Materials and methods
2.1 Slice preparation
Neocortical slices were prepared from Sprague-Dawley rats (17-24 days old). Animals were handled and housed according to the guidelines from the NIH Committee on Laboratory Animal Resources. All experimental protocols were approved by the University of Alabama Institutional Animal Care and Use Committee. Every effort was made to minimize pain and discomfort. Rats were anesthetized with ketamine (100 mg/kg) and decapitated. The brain was removed quickly and placed in ice-cold saline, which contained (in mM): 125 NaCl, 3.5 KCl, 0.5 CaCl2, 3.5 MgCl2, 26 NaHCO3 and 10 D-glucose. The solution was bubbled with 95%O2/5%CO2 to maintain pH around 7.4. Coronal brain slices (300 μm thick), containing frontal association cortex (Paxinos and Watson, 1997), were cut using a Vibratome. The slices were stored for 30 minutes at 37°C and then kept at room temperature in saline similar to that described above but containing 2.5 mM CaCl2 and 1.3 mM MgCl2, until recording. Individual slices were transferred to a recording chamber and continuously perfused (3 ml/min) with oxygenated saline. A Zeiss Axioskop FS (Carl Zeiss, Thornwood, NY) microscope, equipped with Nomarski optics, a 40X-water immersion lens and infrared illumination was used to view neurons in the slices. Layer II/III pyramidal neurons were identified by their pyramidal shape, presence of a prominent apical dendrite, distance from the pial surface and their regular spiking properties.
2.2 Whole-cell recording
Whole-cell voltage-clamp recordings were obtained as described previously (Campbell and Hablitz, 2004). Tight seals (>2 GΩ before breaking into whole-cell mode) were obtained using patch electrodes that had an open tip resistance of around 3 MΩ. Series resistance during recording varied from 10 to 20 MΩ and was not compensated. Recordings were terminated whenever significant increases (>20%) in series resistance occurred. The intracellular solution for recording synaptic currents contained (in mM): 125 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 0.5 EGTA. pH and osmolarity were adjusted to 7.3 and 290 mOsms, respectively. Bicuculline methiodide (BIC) (10 μM) (Sigma-Aldrich St. Louis, MO) was present during all experiments in order to block GABAA receptor mediated IPSCs. Synaptic responses were evoked with a bipolar stimulating electrode (twisted pair of 25 μm Formvar insulated nichrome wires) positioned 150-200 μm below the recording pipette. Stimuli were current pulses 50-100 μA in amplitude and 50-100 μs in duration. A stimulation frequency of 0.05 Hz was used. All traces of synaptic currents shown represent the average of 10 consecutive responses. Recordings were done at 33°C.
2.3 Data collection and analysis
Signals were acquired using an Axon Instruments MultiClamp 700A amplifier (Molecular Devices, Sunnyvale, CA) controlled by Clampex 8.0 software via a Digidata 1200B interface (Molecular Devices). Responses were filtered at 5 kHz, digitized at 10-20 kHz and analyzed using Clampfit 8.0 software (Molecular Devices). Data are expressed as mean ± SEM. Statistical analysis of response amplitudes before, during and after addition of pharmacological agents was carried out using a paired two-tailed Student’s t-test. P<0.05 was considered significant. Miniature EPSCs (mEPSCs) were analyzed off-line using MiniAnalysis software (Synaptosoft, Decatur, GA). Periods of 3 to 5 min were used to calculate values of mEPSC frequency and amplitude under control conditions and during drug application.
2.4 Drug application
Kainate receptor agonists and antagonists were bath applied. After a 10 min control period, kainate and other agents were bath applied for 5-10 min. Acquisition consisted of 15-30 consecutive responses at 0.05 Hz. ATPA, D-APV, CNQX, GYKI52466 and kainate were purchased from Tocris Cookson Inc (Ellisville, MO). LY382884 was a gift from Eli Lilly and Co. Drugs were stored in frozen stock solution and dissolved in the saline prior to each experiment.
3. Results
3.1 Dose-dependent bi-directional effects of kainate on evoked EPSCs
In order to investigate the role of kainate receptors in regulating synaptic responses in neocortex, whole-cell voltage-clamp recordings were obtained from pyramidal cells in layer II/III. Synaptic responses were elicited by intracortical stimulation 150-200 μm below the recording pipette. In the presence of BIC and at a holding potential of -70 mV, small amplitude, presumably AMPA-mediated, EPSCs were evoked with weak stimulation. A typical example of such an EPSC is shown in Fig. 1A. Responses began after a short synaptic delay, had a rapid rising phase and decayed exponentially. Bath application of 250 nM kainate significantly increased EPSC amplitude (191±11 % of control; n=8; p<0.05) with no change in time course (Fig. 1A, upper). The application of 500 nM kainate produced a larger increase in EPSC amplitude (458±80 % of control; n=6; p<0.05; Fig. 1A middle; Fig. 2B). However, when higher concentrations of kainate were used (1 and 3 μM kainate) decreases in EPSC amplitude were seen (Fig. 1A, bottom). Application of 3 μM kainate caused a significant decrease in EPSC amplitude (41±6 % of control; n=3; p<0.05) and also induced a large inward current (see Fig. 7). The effects of 500 nM and 3 μM kainate in a population of neurons (n=6) are summarized in the time course plot shown in Fig. 1B. It can be seen that EPSC amplitude increased rapidly during the 10 min application of 500 nM kainate and partially recovered during the 10 min wash period. Complete recovery was observed in some cells during a longer wash period. These results strongly suggest the presence of high affinity, facilitatory, kainate receptors on excitatory nerve terminals contacting layer II/III pyramidal cells. In contrast, 3 μM kainate produced a slow decrease in EPSC amplitude which did not recover during the wash period. Some recovery was observed in cells recorded for extended periods. As shown in Fig. 1C, EPSC amplitude was increased in the presence of kainate at concentrations ranging from 50 to 500 nM. Increasing the kainate concentration to 1 and 3 μM resulted in a decrease in EPSC amplitude.
Figure 1.
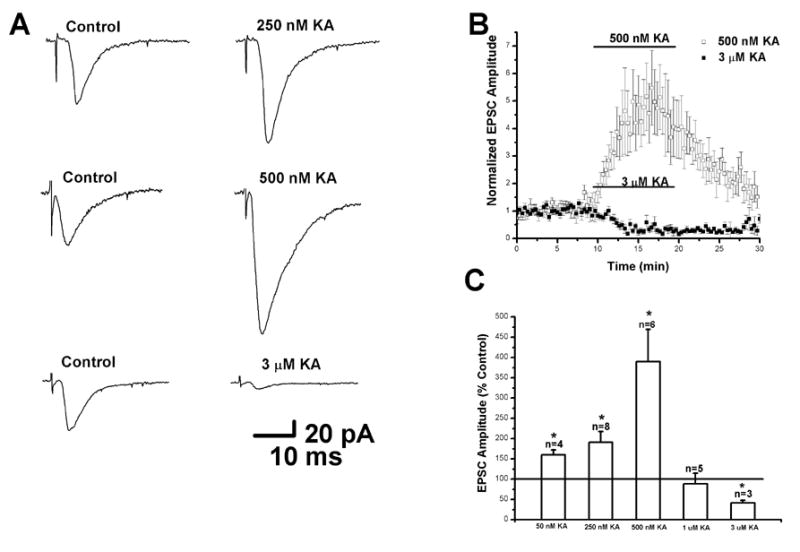
Kainate has dose-dependent bi-directional effects on evoked EPSCs in layer II/III pyramidal cells. (A) Average traces of evoked EPSCs under control conditions (left) and in the presence of 250 nM (top right), 500 nM (middle right) and 3 μM kainate (bottom right). Each trace represents the average of 10 consecutive sweeps. Recordings were obtained in the presence of BIC (10 μM) to block GABAA receptors and cells were held at -70 mV. EPSC amplitudes were enhanced by 250-500 nM kainate and reduced by 3 μM kainate. (B) Plot of evoked EPSC amplitude as a function of time. EPSCs were markedly increased by 500 nM kainate. This effect was partially reversible upon washing. Higher concentrations of kainate (3 μM) depressed EPSC amplitudes. This decrease did not recover during the wash period. (C) Summary bar graphs showing changes in EPSC amplitude induced by different kainate concentrations. (* indicates p < 0.05)
Figure 2.
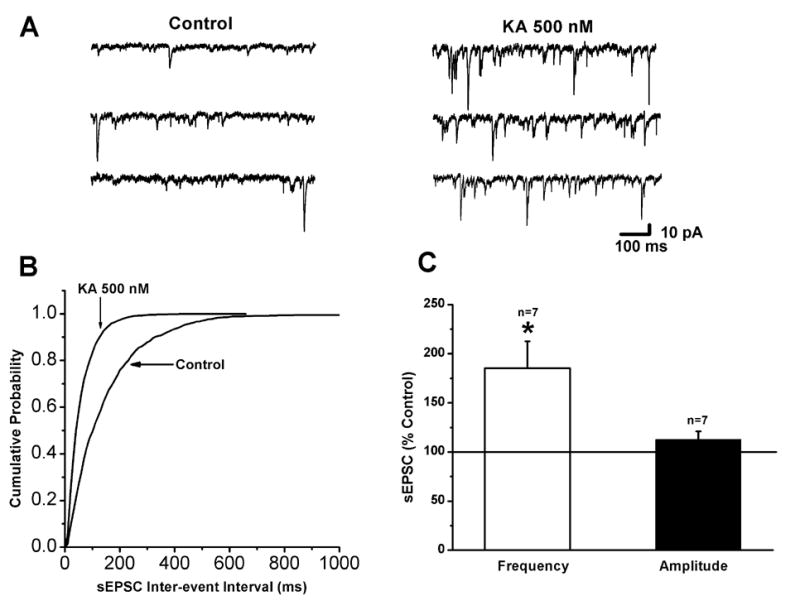
Kainate increases spontaneous EPSC frequency in layer II/III pyramidal cells. (A) Representative traces of spontaneous EPSCs recorded under control conditions (left) and in the presence of 500 nM kainate (right). The frequency of spontaneous EPSCs was markedly increased by kainate. (B) Cumulative distribution plots for sEPSC inter-event intervals showing that kainate produced a leftward shift, indicating an increase in frequency. (C) Bar graphs summarizing the effects of kainate (500 nM) on the frequency and amplitude of sEPSCs. (* indicates p < 0.05)
Figure 7.
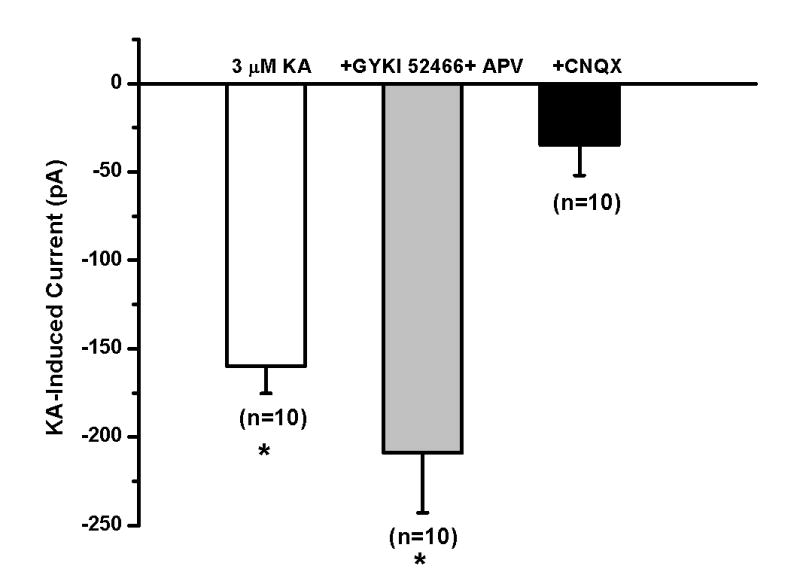
Postsynaptic kainate receptors mediate the inward current produced by high kainate concentration. Summary bar graph showing the inward current induced by kainate (3 μM) during control conditions (left), in the presence of GYKI52466 and D-APV (middle) and in the presence of CNQX (right). Kainate (3 μM) induced a significant inward current under control conditions. GYKI52466 and D-APV did not block the inward current induced by kainate (3 μM). Kainate did not induce a significant inward current in the presence of CNQX (* indicates p<0.05).
3.2 Facilitation of spontaneous EPSCs by kainate
Since the effect of low doses of kainate on evoked EPSCs was quite dramatic, we wished to determine if spontaneous EPSC activity was also facilitated. Examination of specimen records of spontaneous activity under control conditions and after bath application of 500 nM kainate (Fig. 2A, left and right, respectively) clearly indicated an increase in sEPSC frequency. A quantitative analysis of data from the cell in Fig. 2A is shown in Fig. 2B. The cumulative probability plot for inter-event intervals was shifted to the left in the presence of 500 nM kainate, indicating a significant increase in frequency. Results from a group of cells is summarized in Fig. 2C, showing that sEPSC frequency was significantly increased (185±27 % of control; n=7; p<0.05) whereas amplitudes were unaffected (112±8 % of control; n=7; p>0.05). There was no change in the 10-90 % rise time (1.9±0.1 ms in control and 1.8±0.08 ms in kainate; n=7; p>0.05) or decay time constant (3.7±0.3 ms in control and 3.4±0.3 ms in 500 nM kainate; n=7; p>0.05) in the presence of 500 nM kainate.
Kainate receptors have been shown to facilitate EPSCs in hippocampus via a presynaptic mechanism (Schmitz et al., 2001; Contractor et al., 2003). We reasoned that if such a mechanism was involved here, as suggested by the results with sEPSCs, kainate should also affect mEPSCs, increasing their frequency without affecting amplitude. The results of mEPSC experiments are shown in Fig. 3. Specimen records (Fig. 3A) showed that 500 nM kainate significantly increased the frequency of mEPSCs recorded in the presence of 1 μM TTX (178±31 % of control; n=3; p<0.05) whereas amplitudes were unaffected (94±6 % of control; n=3; p>0.5). The increase in frequency was reversible upon washing. The change in frequency is clearly seen in the cumulative probability plot shown in Fig. 3B. Results from experiments with 250 and 500 nM kainate are summarized in Fig. 3C. Kainate at 250 nM also significantly increased mEPSC frequency (142±10 % of control; n=5; p<0.05) without changing amplitudes (106±3 % of control; n=6; p>0.05). There was no change in the kinetics of mEPSCs in the presence of 250 and 500 nM kainate. These results indicate that activation of presynaptic kainate receptors facilitates release of glutamate.
Figure 3.
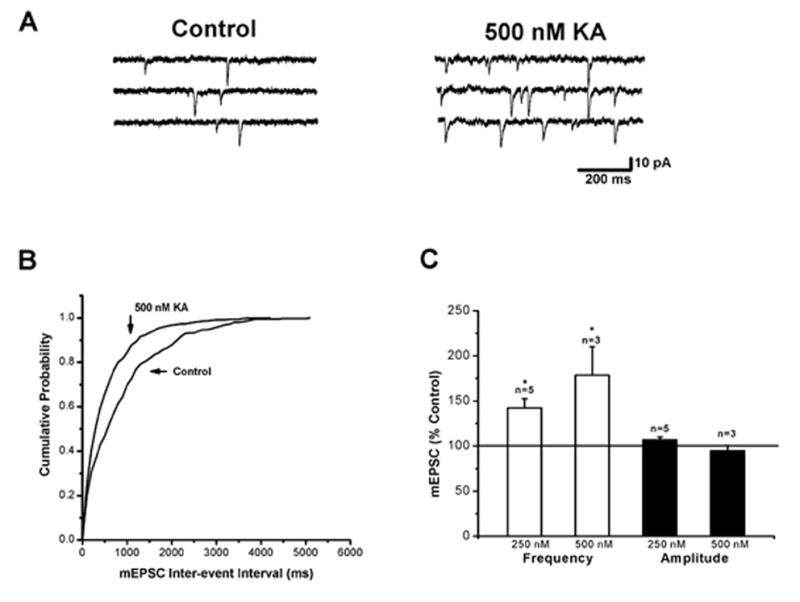
The effect of kainate on miniature EPSCs in layer II/III pyramidal cells. (A) Records of miniature EPSCs under control conditions (left) and in the presence of 500 nM kainate (right). The frequency of miniature EPSCs was increased by kainate. Recordings were obtained in the presence of TTX (1 μM). (B) Cumulative probability plots of inter-event interval of miniature EPSCs. A leftward shift occurred in the presence of kainate, indicative of an increase in frequency. (C) Summary bar graphs of the effects of kainate (250 and 500 nM) on miniature EPSC frequency and amplitude. (* indicates p < 0.05)
Physiologically released glutamate has been shown to modulate excitatory transmission in the hippocampus (Contractor et al., 2001; Lauri et al., 2001) and immature neocortex (Kidd and Issac, 2001; Kidd et al., 2002). To test whether synaptically released glutamate could affect EPSCs in layer II/III pyramidal cells, we examined responses to paired pulse stimulation before and after bath application of the GluR5 antagonist LY382884. It was reasoned that paired pulse facilitation would be decreased following antagonist application due to removal of the facilitatory effect of synaptically released glutamate on kainate receptors. Fig. 4A shows that both control and test EPSCs were decreased by the antagonist. Despite the decrease in the first EPSC amplitude, the second EPSC showed depression rather than facilitation. In a series of cells (n=4) the paired pulse ratio was significantly decreased after application of 10 μM LY382884 (1.1±0.1 in control and 0.68±0.1 in LY382884; n=4; p<0.05) (Fig. 4B). These results indicate that synaptically released glutamate facilitates EPSCs in neocortex.
Figure 4.
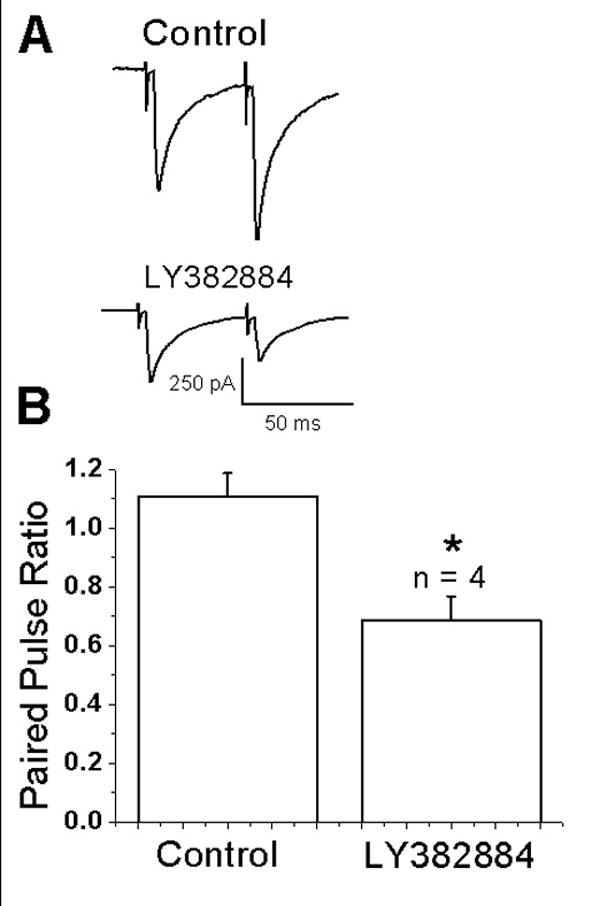
Endogenously released glutamate activates kainate receptors. (A) Trace showing averaged responses (n=10) to a pair of stimuli delivered at a 50 ms interval under control conditions and after application of the GluR5 antagonists LY382884. Both responses were reduced by the antagonist. Paired-pulse facilitation was changed to paired-pulse depression. (B) Summary bar graph showing that under control conditions paired-pulse ratios showed slight facilitation whereas after LY382884 application paired-pulse depression was observed (* indicates p,0.5).
The decreased amplitude of the first response may be due to a facilitatory effect of ambient glutamate, which is known to tonically activate mGluRs in neocortex (Bandrowski et al., 2003). To test this, mEPSCs were recorded before and after bath application of LY382884. It was hypothesized that if presynaptic kainate receptors were tonically active, the antagonist should decrease the frequency of mEPSCs without affecting amplitude. Examination of specimen records of mEPSCs under control conditions (Fig. 5A, left) and in the presence of LY382884 (Fig. 5A, right) indicated that the antagonist decreased the frequency. Quantitative analysis indicated that the frequency was significantly reduced (3.3 ± 0.7 Hz in control and 1.7 ± 0.2 Hz in LY382884; n=4; p<0.05; Fig. 5B) whereas amplitudes (17.2 ± 1.6 pA in control and 19.5 ± 3.0 pA in LY382884; n=4; p>0.05; Fig. 5C) were unaffected. These results indicate that ambient glutamate can tonically activate facilitatory kainate receptors on excitatory nerve terminals.
Figure 5.
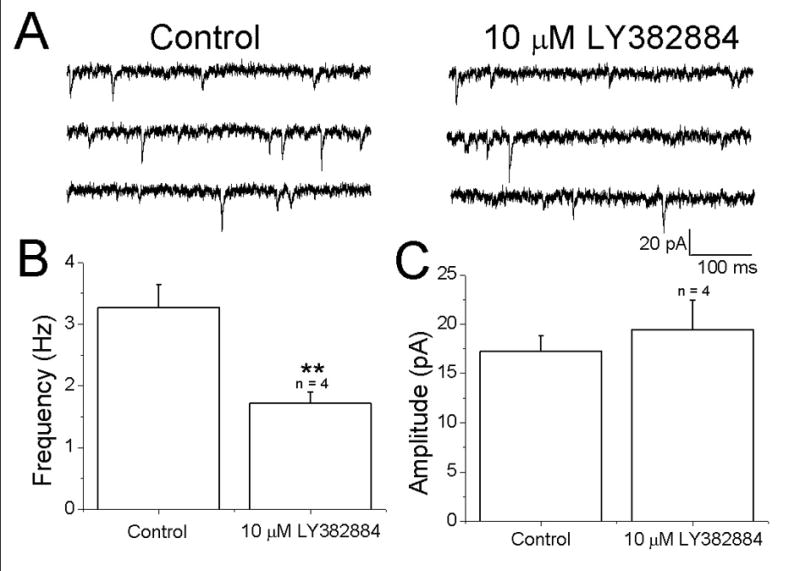
Evidence for tonic activation kainate receptors. (A) Specimen records of mEPSCs in a pyramidal cell under control conditions (left) and after bath application of the GluR5 antagonist LY382884 (right). Visual inspection indicates that the frequency of events was reduced by the antagonist. (B,C) summary of effects of LY382884 on frequency and amplitude.
3.3 Involvement of GluR5 kainate receptors
Multiple kainate receptor subunits are expressed in neocortex (Bahn et al., 1994). The results with LY382884 indicated involvement of GluR5. In order to examine further the mechanisms involved in the facilitatory effect of kainate and to determine the role of GluR5 subunit-containing receptors, the effects of the specific GluR5 agonist ATPA on evoked and mEPSCs were determined. As shown in the specimen records in Fig. 6A, evoked EPSC amplitudes were significantly increased in the presence of 1 μM ATPA (218±39 % of control, n=4, p<0.05, Fig. 6C). When 10 μM ATPA was applied (Fig. 6B), EPSC amplitudes were reduced by 80±4 % (n=6, p<0.05, Fig. 6D). An increase in holding current was also observed with 10 (2378±945 % of control) but not 1 μM (98±26% of control) ATPA (Fig. 6 C and D). These results suggest a role for GluR5 subunit containing receptors in facilitation of excitatory transmission in layer II/III pyramidal cells. After 10 min of exposure to 10 μM ATPA, membrane noise was significantly increased and mEPSCs could not be detected. This was reversible upon washing. Similar results were seen in all cells tested (n=5).
Figure 6.
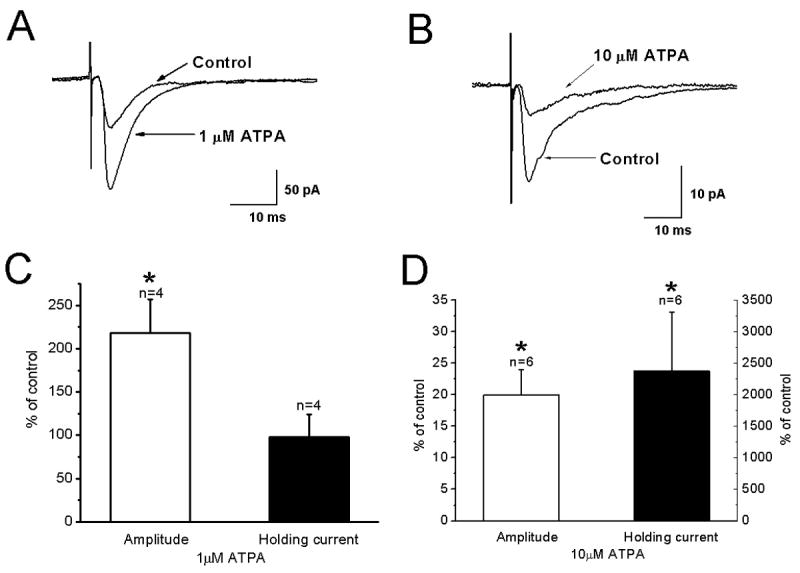
Effects of the GluR5 agonist ATPA on evoked EPSC amplitudes and mEPSC frequency. (A) Average traces of eEPSCs under control conditions and in the presence of ATPA (1 μM) showing ATPA enhanced EPSC amplitudes. (B) Similar to A but showing that 10 μM ATPA decreased EPSC amplitudes. (C) Summary bar graph of the effect of 1 μM ATPA on EPSC amplitude (left) and holding current (right). (D) Summary of effects of 10 μM ATPA on EPSC amplitude and holding current.
3.4 Postsynaptic effects of kainate
As noted above, kainate and ATPA, at higher concentrations, induced an inward current. Two sets of experiments were performed to analyze the mechanisms involved. To investigate if presynaptic kainate receptor activation induces glutamate release which subsequently acts on postsynaptic NMDA- and/or AMPA-receptors to produce an inward current, kainate was applied in the presence of TTX and glutamate receptor antagonists. As shown in Fig. 7, 3 μM kainate induced an inward current (-162±21 pA; n=3; p<0.05) under control conditions. In the presence of 50 μM D-APV and 50 μM GYKI52466 to block NMDA and AMPA receptors, respectively, and TTX to block sodium-dependent action potentials, 3 μM kainate still induced an inward current (-208±34 pA; n=10; p<0.05). These results indicate that the inward current was not due to activation of postsynaptic AMPA and NMDA receptors. Alternatively, at high concentrations, kainate could act on postsynaptic kainate receptors to induce an inward current. In the presence of 20 μM CNQX to block kainate receptors, no significant change in holding current (mean holding current change: -35±17 pA; n=10; p>0.05) was observed after application of 3 μM kainate. These results suggest that at higher concentrations kainate and ATPA activate postsynaptic kainate receptors.
3.5 Kainate receptor mediated EPSCs
The block of the kainate-induced inward current by CNQX suggests the presence of postsynaptic kainate receptors. Previous studies have suggested that postsynaptic kainate receptor mediated EPSCs are present in neonatal layer IV neurons (Kidd and Isaac, 2001) and layer V pyramidal cells (Eder et al., 2003c). To test for the presence of kainate-mediated postsynaptic currents in layer II/III pyramidal cells, EPSCs were recorded in the presence of BIC (10 μM), GYKI52466 (50 μM) and D-APV (50 μM). An example of an EPSC evoked under these conditions is shown in Fig. 8A. The addition of CNQX (20 μM) significantly reduced the response, indicating mediation by kainate receptors. As shown in Fig. 8B, application of CNQX caused a significant decrease in EPSCs evoked in the presence of D-APV, GYKI52466 and BIC (33±8 pA control vs 4±2.2 pA CNQX; n=12; p<0.05). EPSCs rise times recorded in control saline and in the presence of D-APV, GYKI52466 and BIC were not significantly different (Fig. 8C). Decay times were significantly longer in the presence of D-APV and GYKI52466 (Fig. 8D), indicating that the response was not due to unblocked AMPA receptors. These results suggest that synaptically released glutamate activates postsynaptic kainate receptors that contribute to synaptic transmission in layer II/III pyramidal cells.
Figure 8.
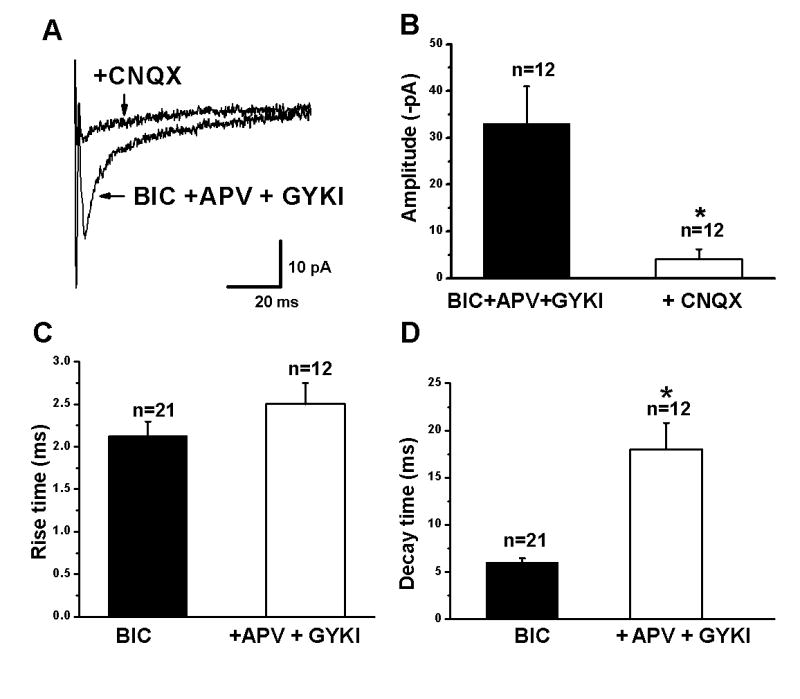
Kainate mediated EPSCs in layer II/III pyramidal neurons. (A) Averaged trace of EPSCs evoked in the presence of 10 μM bicuculline (BIC), D-APV (50 μM) and GYKI52466 (50 μM) and after addition of CNQX (20 μM). (B) Summary graph showing that CNQX blocks the EPSC recorded in the presence of BIC, APV and GYKI, indicating mediation by kainate receptors. (C,D) Summary of effects of APV and GYKI application on EPSC rise and decay times, respectively. Rise times were not affected whereas decay times were significantly prolonged (* indicates p<0.05).
4. Discussion
We demonstrated here that activation of kainate receptors produces dose-dependent biphasic effects on EPSCs in layer II/III neocortical pyramidal cells. An enhancement of evoked EPSC amplitudes and increases in the frequency of both spontaneous and miniature EPSCs were observed at low kainate concentrations. The increase in the frequency but not the amplitude of mEPSCs indicates that facilitation was via a presynaptic mechanism. EPSC depression and a postsynaptic current were seen at higher concentrations. The increase in holding current during application of 1-3 μM kainate and the ability to evoke an EPSC in the presence of D-APV and GYKI52466 indicate the presence of postsynaptic kainate receptors in layer II/III pyramidal cells. The GluR5 subunit specific agonist ATPA mimicked the facilitatory effects of kainate on both evoked and spontaneous EPSCs suggesting mediation by receptors containing this subtype. These results indicate that functional GluR5-containing kainate receptors are present on both sides of excitatory synapses in the neocortex and play an important role in synaptic transmission.
4.1 Bi-directional effect of kainate on eEPSCs in layer II/III pyramidal cells
Kainate has bi-directional, dose-dependent effects at hippocampal mossy fiber synapses, causing an increase in EPSC amplitudes at lower concentrations (Schmitz et al., 2001; Contractor et al., 2003) and decreases at higher concentrations (Kamiya and Ozawa, 2000; Contractor et al., 2000; Schmitz et al., 2001). Similar results have been reported at parallel fiber synapses in the cerebellar cortex (Delaney and Jahr, 2002). In contrast, synaptic transmission in the hippocampal CA1 region is generally depressed by kainate (Clarke and Collingridge, 2002; Breustedt and Schmitz, 2004) although bidirectional effects have been observed (Chittajallu et al., 1996). At the hippocampal mossy fiber-CA3 pyramidal cell synapse, it has been suggested that an ionotropic depolarizing action of kainate is responsible for both facilitation and depression (Schmitz et al., 2001). Our results suggest that eEPSCs in neocortex are facilitated at low doses via a presynaptic mechanism whereas depression may involve activation of postsynaptic receptors. Facilitation at low kainate concentrations could be due to the presence of heteromeric GluR5 receptors with higher affinity subunits at presynaptic sites (e.g., KA2, Contractor et al., 2003; Ruiz et al., 2005) or higher density of receptors in the terminal.
In addition to decreasing evoked EPSC amplitudes, kainate, at 1-3 μM, produced a significant increase in holding current. The changes in holding current were sensitive to CNQX, but not D-APV and GYKI52466, indicating mediation by kainate receptors. The persistence of a kainate-activated inward current in the presence of D-APV and GYKI52466 suggests that kainate is directly activating postsynaptic receptors. The mechanism underlying the depression of evoked EPSCs is not clear. Activation of postsynaptic receptors could lead to decreased responsiveness due to shunting, altered dendritic-somatic coupling, or desensitization (Rozov et al., 2001).
4.2 Kainate enhances the frequency of spontaneous and miniature EPSCs in layer II/III pyramidal cells
Presynaptic kainate receptors have been shown to facilitate both evoked EPSCs (Schmitz et al., 2001; Breustedt and Schmitz, 2004) and IPSCs (Cossart et al., 2001; Braga et al., 2003). The effects of kainate on mEPSCs and mIPSCs are less clear. Increases in mEPSCs frequency in hippocampal CA3 neurons have been reported in some (Contractor et al., 2000) but not all (Castillo et al., 1997) studies. The reported effects of kainate on mIPSCs have also been variable. At interneuron to CA1 pyramidal cell synapses in the hippocampus, either no change (Frerking et al., 1998) or decreases (Rodriguez-Moreno et al., 1997; Cossart et al., 1998) in mIPSC frequency upon kainate application have been reported. At the dorsal horn inhibitory synapse in the spinal cord, kainate application increased the frequency of mIPSCs (Kerchner et al., 2001). Other recent studies have also reported an increase in synaptic transmission using lower concentrations of kainate on mEPSCs (Contractor et al., 2000) and mIPSCs (Cossart et al., 2001; Nakamura et al., 2003). There are several factors that could contribute to the discrepancy regarding the effect of kainate on mPSCs. These include the concentration of kainate, type of synapse, kainate receptor subunit expression, or kainate’s mechanism of action. Our results in layer II/III pyramidal cells demonstrate that presynaptic kainate receptors modulate glutamate release by increasing the frequency of mEPSCs.
4.3 Kainate facilitation involves GluR5 subunits
The GluR5 kainate receptor subunit specific agonist ATPA mimicked the facilitatory effects of kainate on both evoked and mEPSCs. GluR5 subunits are capable of forming functional homomeric ligand-gated channels when expressed in isolation and can co-assemble with all other KAR subunits including GluR6 to form heteromeric channels (Cui and Mayer, 1999; Bortolotto et al., 1999; Paternain et al., 2000). ATPA can activate both homomeric GluR5 and heteromeric GluR5-containing functional receptors (Cui and Mayer, 1999; Paternain et al., 2000). Our results with ATPA strongly suggest the involvement of GluR5-containing receptors but do not uniquely identify the subunit composition of the receptors involved.
In the hippocampus, the nature of the kainate receptor subunits underlying modulation of mossy fiber EPSCs has not been firmly established. Studies using an antagonist selective for GluR5-containing receptors suggested a role for these receptors (Bortolotto et al., 1999; Lauri et al., 2001, 2003; Zuner et al., 2003) whereas reports using knock-out mice have indicated that GluR6-containing receptors are involved in modulation of mossy fiber synapses (Contractor et al., 2001; Breustedt and Schmitz, 2004; Schmitz et al., 2005). This latter result is consistent with the low level of GluR5 expression in granule cells (Bahn et al., 1994; Paternain et al., 2000) and the presence of GluR6 subunits at mossy fiber terminals (Darstein et al., 2003). Our data implicate GluR5-containing receptors in facilitation of neocortical EPSCs, suggesting that there are regional differences in subunits mediating kainate facilitation. The results with the GluR5 antagonist LY382884 suggest that ambient levels of glutamate can tonically activate these facilitatory receptors.
Synaptically released glutamate, acting on kainate receptors, has been shown to modulate excitatory transmission in the hippocampus (Contractor et al., 2001; Lauri et al., 2001) and immature neocortex (Kidd and Issac, 2001; Kidd et al., 2002). In the present study we found that bath application of the GluR5 antagonist LY382884 decreased EPSC amplitudes and changed paired-pulse facilitation to paired-pulse depression. We attribute the effects on the first EPSCs during paired stimulation to block of kainate receptors tonically activated by ambient glutamate. Tonic activation of presynaptic receptors has been reported for both GABA-ergic (Stell and Mody 2002; Semyanov et al. 2003; Keros and Hablitz 2005) and glutamatergic synapses (Sah et al., 1989; Bandrowski et al., 2003; Cavelier and Attwell 2005). The block of facilitation of the second response can be attributed to loss of a facilitatory effect of evoked glutamate release. During paired-pulse or repetitive activation in the neocortex, excitatory synapses show frequency-dependent depression (Thomson et al., 1993; Markram and Tsodyks, 1996; Varela et al., 1997), as observed after LY382884 application.
4.4 Postsynaptic kainate receptors
EPSCs mediated by postsynaptic kainate receptors have been described at synapses in hippocampus (Frerking and Nicoll, 2000; Lerma et al., 2001; Cossart et al., 2002) and neocortex (Kidd and Isaac, 1999, 2001; Eder et al., 2003). Kainate-receptor mediated EPSCs often have small amplitudes, slow kinetics and may require repetitive synaptic stimulation for activation (Vignes and Collingridge, 1997; Castillo et al., 1997) raising questions about the contribution of kainate receptors to normal glutamatergic transmission. In the present experiments, EPSCs could be evoked by single shock stimulation in the presence of D-APV and GYKI52466, displaying kinetics similar to control responses. Our results and those of others (Eder et al., 2003) suggest that kainate EPSCs are reliably evoked in neocortical neurons along with AMPA- and NMDA-mediated responses. The source of the afferent fibers responsible for kainate mediated EPSCs is presently unclear and it will be important to compare thalamocortical and intracortical inputs to pyramidal cells.
The ability of single shock stimulation to evoke a composite EPSC consisting of AMPA and kainate components suggests that the sensitivity of these postsynaptic receptors to synaptically released glutamate may be similar. The similar kinetics suggests that kainate receptors may be located at the synapse, consistent with the lack of effect of uptake blockers (Bureau et al., 2000; Kidd and Isaac, 2001). The presence of facilitatory autoreceptors in the neocortex has been implicated (Perkinton and Sihra, 1999). Our studies support the presence of facilitatory presynaptic kainate receptor. Extrasynaptic kainate receptors have been described on cortical pyramidal neurons (Eder et al., 2003) and may underlie the inward current induced by 1-3 μM kainate.
In summary, we have provided evidence that presynaptic kainate receptors in layer II/III pyramidal cells act to facilitate glutamate release and modulate synaptic transmission in response to exogenous application of kainate and kainate receptor agonists. These effects appear to involve GluR5 subunit-containing receptors. Postsynaptic kainate receptor mediated EPSCs also contribute to synaptic activation of layer II/III pyramidal cells suggesting multiple roles for kainate receptors in synaptic transmission in neocortex.
Acknowledgments
This work was supported by NIH grant NS22373 and P30 NS47466.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ali AB, Rossier J, Staiger JF, Audinat E. Kainate receptors regulate unitary IPSCs elicited in pyramidal cells by fast-spiking interneurons in the neocortex. J Neurosci. 2001;21:2992–2999. doi: 10.1523/JNEUROSCI.21-09-02992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB. Involvement of post-synaptic kainate receptors during synaptic transmission between unitary connections in rat neocortex. Eur J Neurosci. 2003;17:2344–2350. doi: 10.1046/j.1460-9568.2003.02677.x. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandrowski AE, Huguenard JR, Prince DA. Baseline glutamate levels affect group I and II mGluRs in layer V pyramidal neurons of rat sensorimotor cortex. J Neurophysiol. 2003;89:1308–1316. doi: 10.1152/jn.00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Mulle C. Review: Neurotransmitter Receptors II: AMPA and Kainate Receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VRJ, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are invovled in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Braga MFM, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional Modulation of GABA Release by Presynaptic Glutamate Receptor 5 Kainate Receptors in the Basolateral Amygdala. J Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt J, Schmitz D. Assessing the Role of GLUK5 and GLUK6 at Hippocampal Mossy Fiber Synapses. J Neurosci. 2004;24:10093–10098. doi: 10.1523/JNEUROSCI.3078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. PNAS. 2000;97:6838–6843. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Hablitz JJ. Glutamate transporters regulate excitability in local networks in rat neocortex. Neuroscience. 2004;127:625–635. doi: 10.1016/j.neuroscience.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. Journal of Physiology (Lond) 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VRJ, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Clarke VRJ, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas JP, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Clarke VRJ, Collingridge GL. Characterisation of the effects of ATPA, a GLUK5 receptor selective agonist, on excitatory synaptic transmission in area CA1 of rat hippocampal slices. Neuropharmacology. 2002;42:889–902. doi: 10.1016/s0028-3908(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of Kainate Receptor-Mediated Heterosynaptic Facilitation of Mossy-Fiber Synapses in KA2-/- Mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O’Gorman S, Heinemann SF. Identification of the Kainate Receptor Subunits Underlying Modulation of Excitatory Synaptic Transmission in the CA3 Region of the Hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crepel V. Quantal release of glutamate generates pure kainate and mixed AMPA/Kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29:497–508. doi: 10.1016/s0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassmebly of GluR5, GluR6, and GluR7. J Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of Kainate Receptor Subunits at Hippocampal Mossy Fiber Synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–482. doi: 10.1016/s0896-6273(02)01008-5. [DOI] [PubMed] [Google Scholar]
- Eder M, Becker K, Rammes G, Schierloh A, Azad SC, Zieglgansberger W, Dodt HU. Distribution and Properties of Functional Postsynaptic Kainate Receptors on Neocortical Layer V Pyramidal Neurons. J Neurosci. 2003;23:6660–6670. doi: 10.1523/JNEUROSCI.23-16-06660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg P. The KA-2 subunit of excitatory amino acid receptors show widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned Glutamate Receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol. 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic Kainate Receptors Regulate Spinal Sensory Transmission. J Neurosci. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Coumis U, Collingridge GL, Crabtree JW, Isaac JTR. A presynaptic kainate receptor is invovlved in regulating the dynamic properties of thalamocortical synapses during development. Neuron. 2002;34:635–646. doi: 10.1016/s0896-6273(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JTR. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JTR. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses:role of glutamate clearance. J Neurophysiol. 2001;86:1139–1148. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JTR, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JTR, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular Physiology of Kainate Receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Li H, Chen A, Xing G, Wei M-L, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nature Neurosci. 2001;4:612–620. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Jang IS, Ishibashi H, Watanabe S, Akaike N. Possible Roles of Kainate Receptors on GABAergic Nerve Terminals Projecting to Rat Substantia Nigra Dopaminergic Neurons. J Neurophysiol. 2003;90:1662–1670. doi: 10.1152/jn.01165.2002. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 Kainate Receptor Subunits Coexist in Hippocampal Neurons and Coassemble to Form Functional Receptors. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Perkinton MS, Sihra TS. A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes) Neuroscience. 1999;90:1281–1292. doi: 10.1016/s0306-4522(98)00573-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA Receptor Channels with Long-Lasting Desensitization in Bipolar Interneurons Contribute to Synaptic Depression in a Novel Feedback Circuit in Layer 2/3 of Rat Neocortex. J Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nature Neurosci. 2005;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. PNAS. 2001;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal. J Neurosci. 2002;22(RC223):1–5. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Clarke VRJ, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology. 1998;37:1269–1277. doi: 10.1016/s0028-3908(98)00148-8. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high affinity kainate receptor expressed predominately in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Differential anatgonism of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol Pharmacol. 1995;47:582–587. [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuner AB, Sari L, John TRI, Graham LC. Kainate receptors and the induction of mossy fibre long-term potentiation. Philosophical T Roy Soc B. 2003;358:657–666. doi: 10.1098/rstb.2002.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]


