Abstract
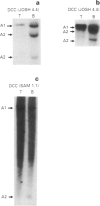
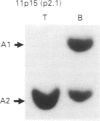
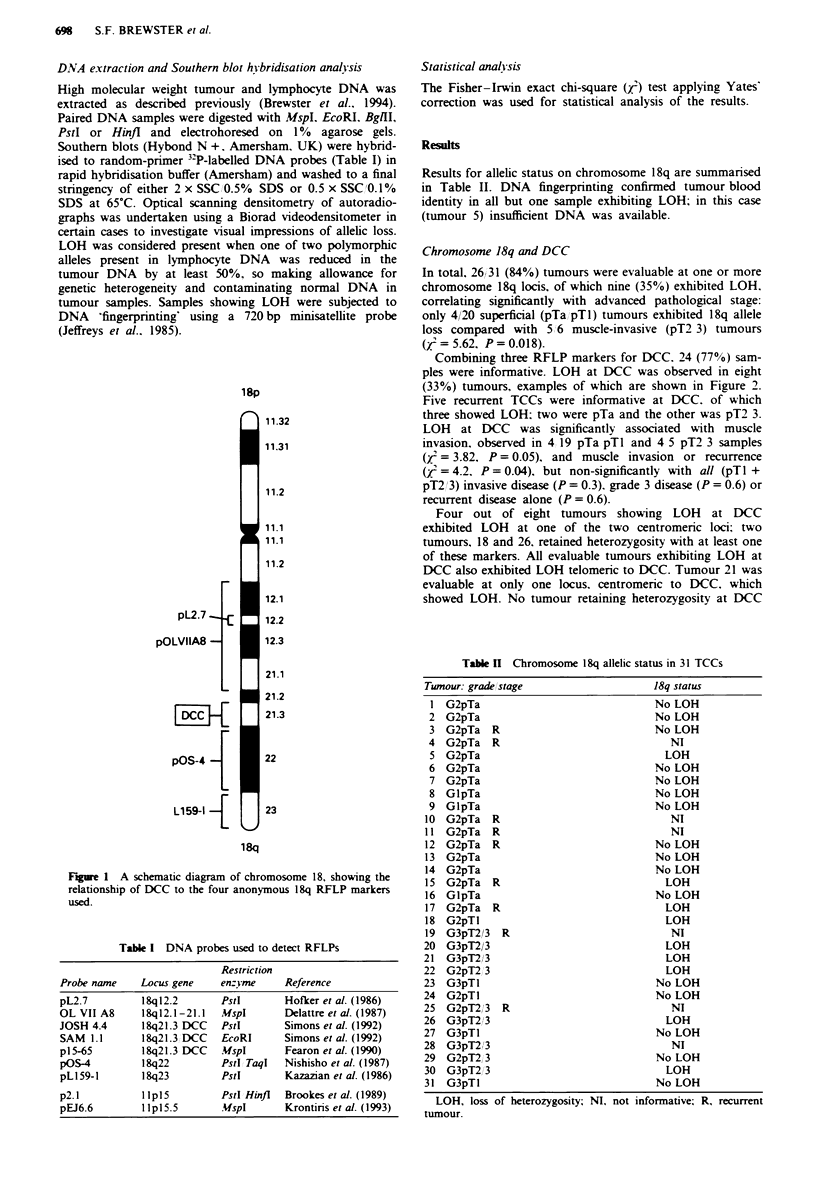
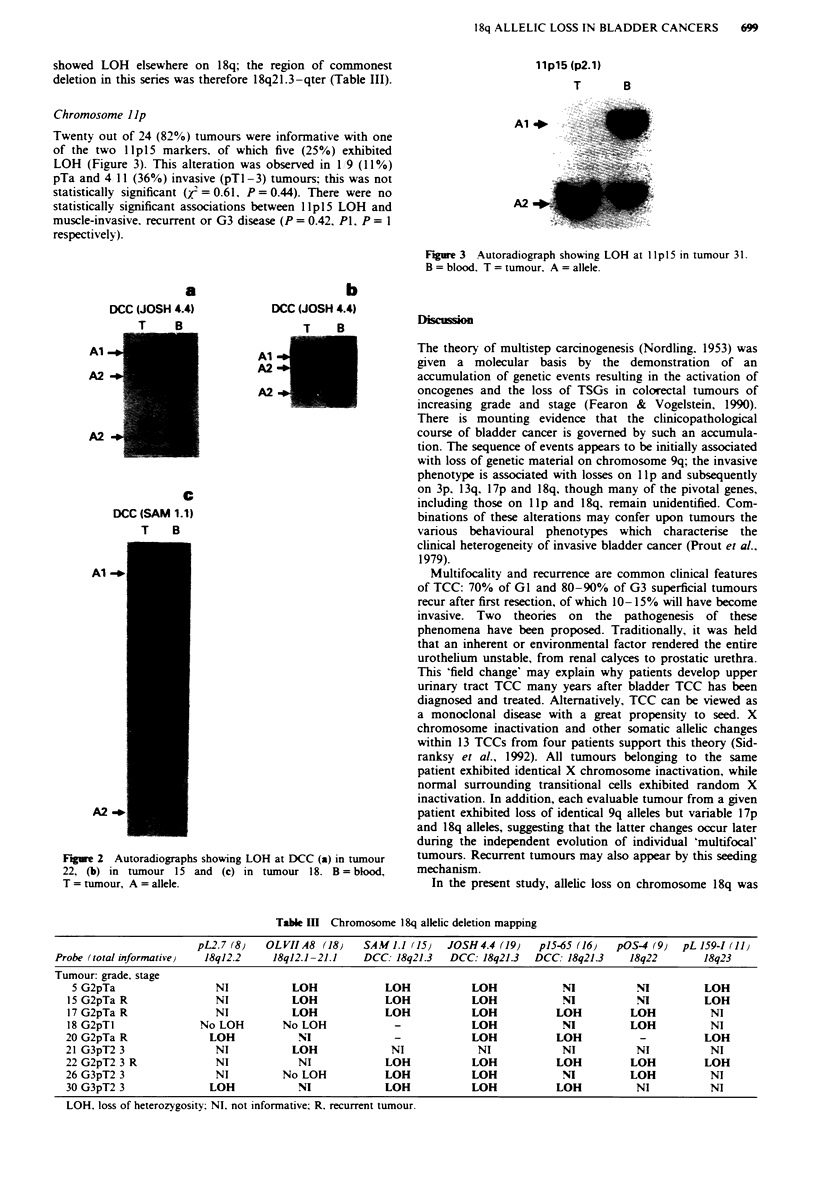
Somatic allelic loss is regarded as a hallmark of tumour-suppressor gene (TSG) inactivation. Thirty-one human bladder transitional cell carcinomas (TCCs) were examined for allelic loss at five chromosome 18q loci, including the DCC gene (deleted in colorectal carcinoma) and at chromosome 11p15 in a restriction fragment length polymorphism analysis. Allelic loss was observed at one or more 18q loci in 9/26 (35%) samples, associated with muscle-invasive disease (P < 0.02). Allelic loss was observed at DCC in 8/24 (33%) samples, associated with muscle-invasive disease (P = 0.05). Three out of the five evaluable recurrent TCCs exhibited allelic loss at DCC, two of which were superficial. No allelic losses were detected at other 18q loci in tumours which retained both DCC alleles. Allelic loss was observed at 11p15 in 5/20 (25%) tumours. These data suggest the presence of a late-acting TSG located on 18q in TCC bladder cancer. DCC is a candidate gene since it lies within the region of most common deletion (18q21.3-qter).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewster S. F., Browne S., Brown K. W. Somatic allelic loss at the DCC, APC, nm23-H1 and p53 tumor suppressor gene loci in human prostatic carcinoma. J Urol. 1994 Apr;151(4):1073–1077. doi: 10.1016/s0022-5347(17)35186-8. [DOI] [PubMed] [Google Scholar]
- Brewster S. F., Gingell J. C., Brown K. W. Tumour suppressor genes in urinary tract oncology. Br J Urol. 1992 Dec;70(6):585–590. [PubMed] [Google Scholar]
- Brewster S. F., Simons J. W. Gene therapy in urological oncology: principles, strategies and potential. Eur Urol. 1994;25(3):177–182. doi: 10.1159/000475279. [DOI] [PubMed] [Google Scholar]
- Brookes A. J., Hedge P. H., Solomon E. A highly polymorphic locus on chromosome 11 which has homology to a collagen triple-helix coding sequence. Nucleic Acids Res. 1989 Feb 25;17(4):1792–1792. doi: 10.1093/nar/17.4.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns P., Proctor A. J., Knowles M. A. Loss of heterozygosity at the RB locus is frequent and correlates with muscle invasion in bladder carcinoma. Oncogene. 1991 Dec;6(12):2305–2309. [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Initiation of bladder cancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene. 1993 Apr;8(4):1083–1085. [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chenevix-Trench G., Leary J., Kerr J., Michel J., Kefford R., Hurst T., Parsons P. G., Friedlander M., Khoo S. K. Frequent loss of heterozygosity on chromosome 18 in ovarian adenocarcinoma which does not always include the DCC locus. Oncogene. 1992 Jun;7(6):1059–1065. [PubMed] [Google Scholar]
- Dalbagni G., Presti J., Reuter V., Fair W. R., Cordon-Cardo C. Genetic alterations in bladder cancer. Lancet. 1993 Aug 21;342(8869):469–471. doi: 10.1016/0140-6736(93)91595-d. [DOI] [PubMed] [Google Scholar]
- Delattre O., Bernard A., Marlhens F., Monpezat J. P., Dutrillaux B., Thomas G. RFLP identified by the anonymous DNA segment OL VII A8 at 18q11 (HGM8 no. D18S7). Nucleic Acids Res. 1987 Feb 11;15(3):1343–1343. doi: 10.1093/nar/15.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Habuchi T., Ogawa O., Kakehi Y., Ogura K., Koshiba M., Hamazaki S., Takahashi R., Sugiyama T., Yoshida O. Accumulated allelic losses in the development of invasive urothelial cancer. Int J Cancer. 1993 Feb 20;53(4):579–584. doi: 10.1002/ijc.2910530409. [DOI] [PubMed] [Google Scholar]
- Hofker M. H., Skraastad M. I., Bergen A. A., Wapenaar M. C., Bakker E., Millington-Ward A., van Ommen G. J., Pearson P. L. The X chromosome shows less genetic variation at restriction sites than the autosomes. Am J Hum Genet. 1986 Oct;39(4):438–451. [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Boehm C. D., Goff S. C., Wong C., Dowling C. E., Newburger P. E., Knowlton R. G., Brown V., Donis-Keller H. Characterization of a spontaneous mutation to a beta-thalassemia allele. Am J Hum Genet. 1986 Jun;38(6):860–867. [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Devlin B., Karp D. D., Robert N. J., Risch N. An association between the risk of cancer and mutations in the HRAS1 minisatellite locus. N Engl J Med. 1993 Aug 19;329(8):517–523. doi: 10.1056/NEJM199308193290801. [DOI] [PubMed] [Google Scholar]
- NORDLING C. O. A new theory on cancer-inducing mechanism. Br J Cancer. 1953 Mar;7(1):68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Galan T., Fair W. R., Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–5409. [PubMed] [Google Scholar]
- Prout G. R., Jr, Griffin P. P., Shipley W. U. Bladder carcinoma as a systemic disease. Cancer. 1979 Jun;43(6):2532–2539. doi: 10.1002/1097-0142(197906)43:6<2532::aid-cncr2820430654>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Kao C., Messing E. M., Newton M., Swaminathan S. A molecular genetic model of human bladder carcinogenesis. Semin Cancer Biol. 1993 Jun;4(3):143–152. [PubMed] [Google Scholar]
- Sidransky D., Frost P., Von Eschenbach A., Oyasu R., Preisinger A. C., Vogelstein B. Clonal origin bladder cancer. N Engl J Med. 1992 Mar 12;326(11):737–740. doi: 10.1056/NEJM199203123261104. [DOI] [PubMed] [Google Scholar]
- Simons J. W., Oliner J. D., Cho K. R., Kinzler K. W., Vogelstein B. SAM 1.1 and JOSH 4.4: two RFLPs within the human DCC gene. Hum Mol Genet. 1992 Aug;1(5):352–352. doi: 10.1093/hmg/1.5.352. [DOI] [PubMed] [Google Scholar]
- Thompson A. M., Morris R. G., Wallace M., Wyllie A. H., Steel C. M., Carter D. C. Allele loss from 5q21 (APC/MCC) and 18q21 (DCC) and DCC mRNA expression in breast cancer. Br J Cancer. 1993 Jul;68(1):64–68. doi: 10.1038/bjc.1993.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. C., Nichols P. W., Hiti A. L., Williams Z., Skinner D. G., Jones P. A. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Res. 1990 Jan 1;50(1):44–47. [PubMed] [Google Scholar]
- Uchino S., Tsuda H., Noguchi M., Yokota J., Terada M., Saito T., Kobayashi M., Sugimura T., Hirohashi S. Frequent loss of heterozygosity at the DCC locus in gastric cancer. Cancer Res. 1992 Jun 1;52(11):3099–3102. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]