Abstract
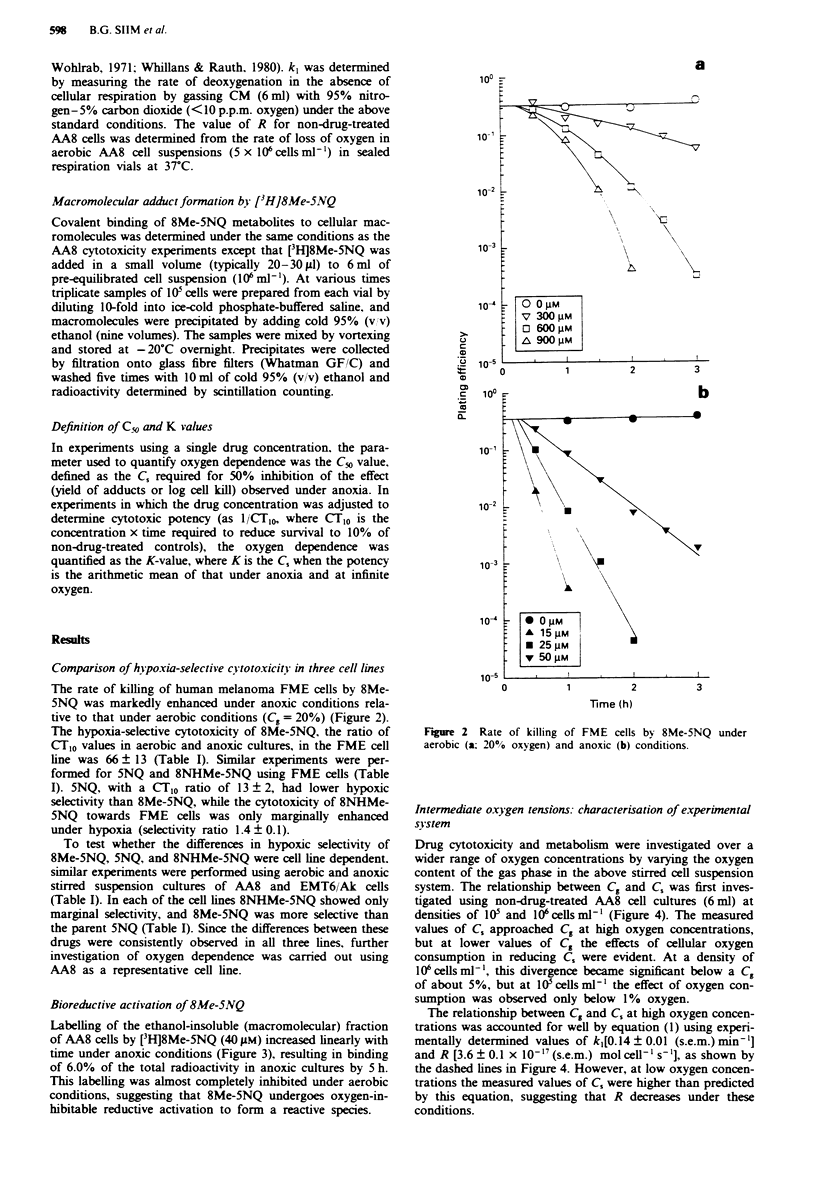
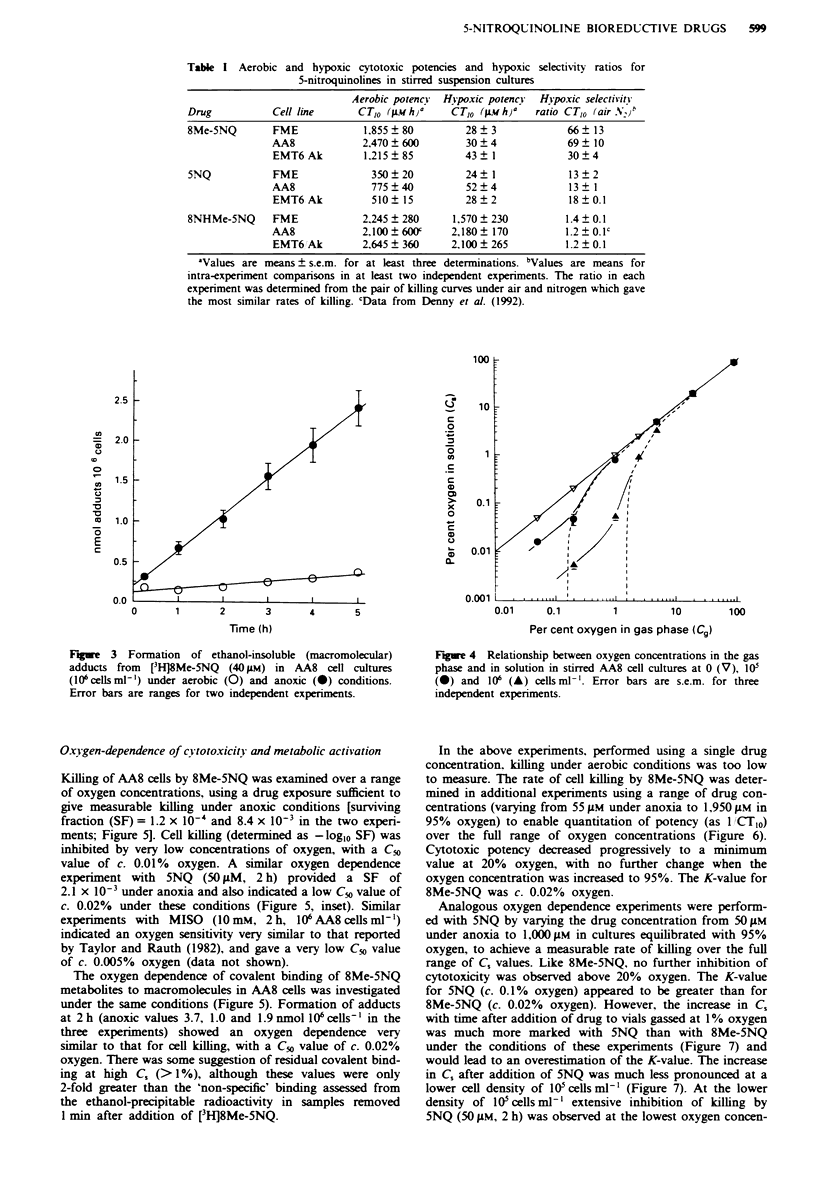
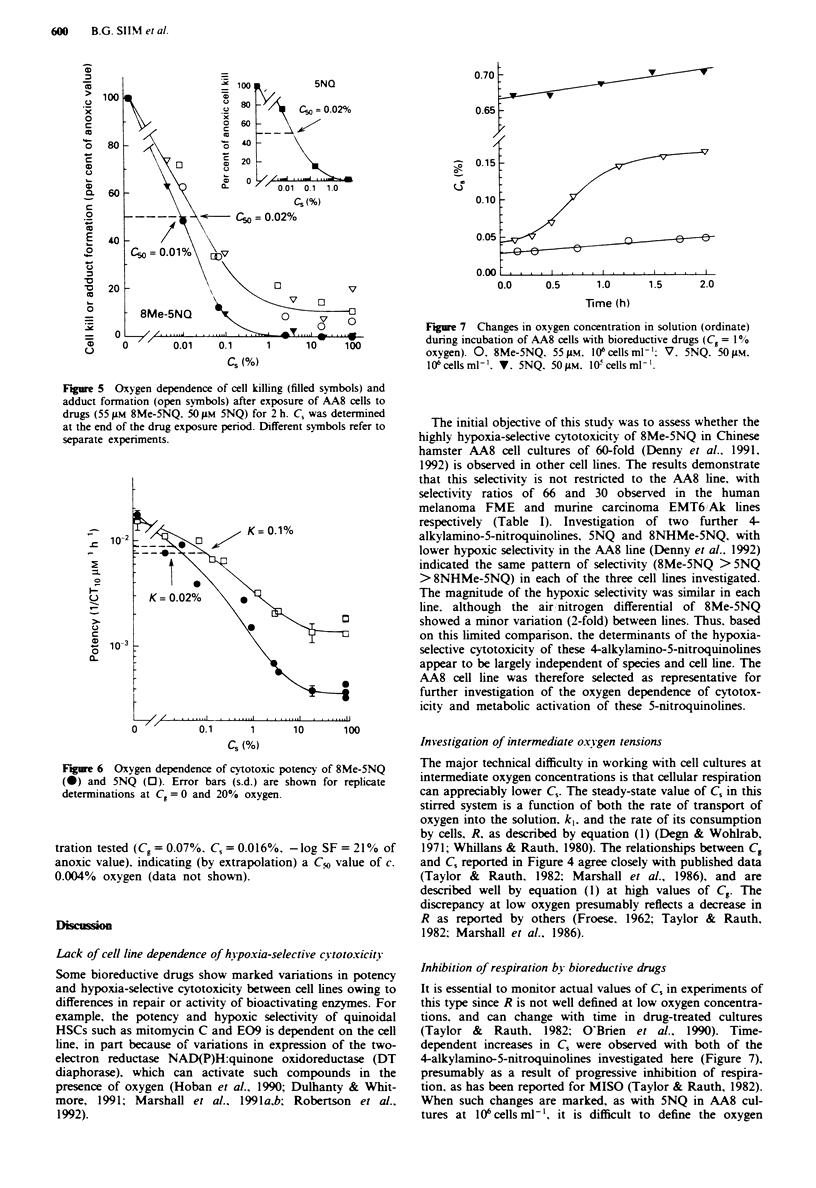
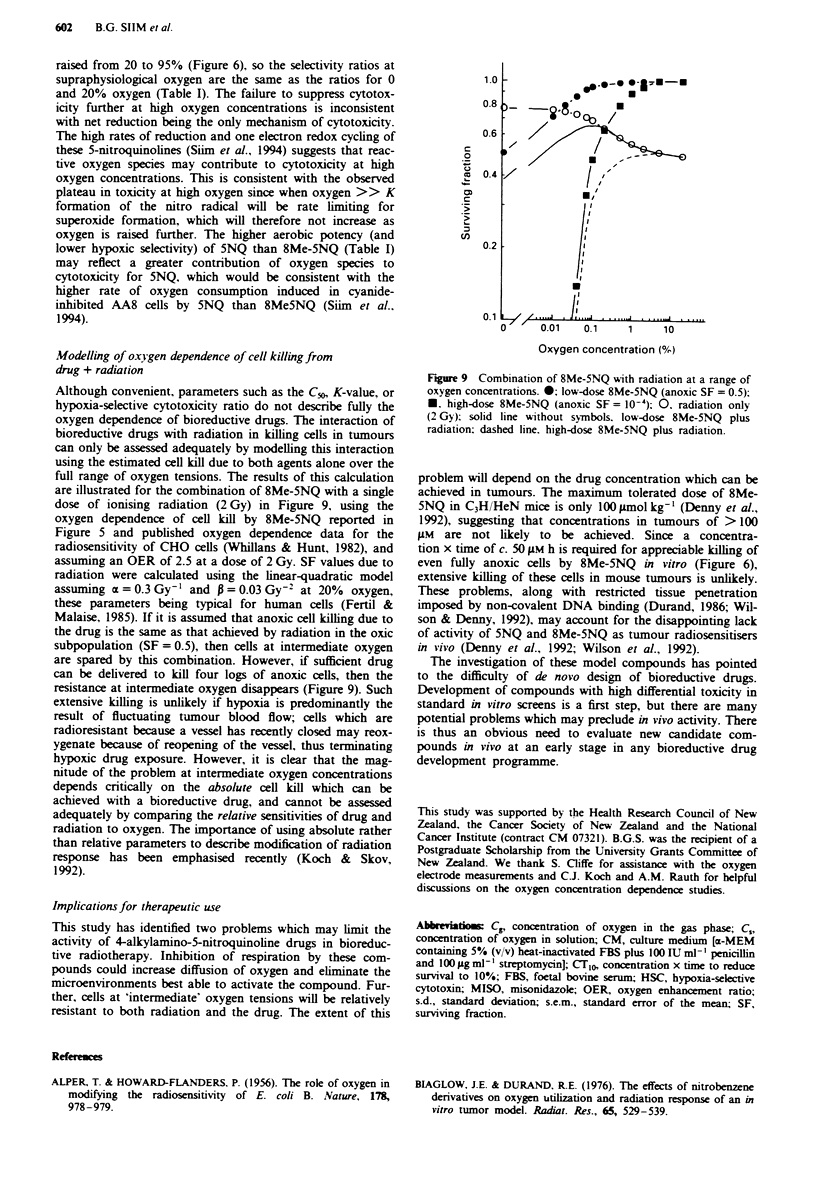
The cytotoxic potency of 4-alkylamino-5-nitroquinoline drugs in AA8 cell cultures is enhanced up to 60-fold under hypoxia, with wide variations in selectivity for hypoxic cells observed for different members of this series. This study uses three representative 5-nitroquinolines to examine whether these differences in hypoxia-selective cytotoxicity are cell line specific, and to explore quantitatively the oxygen dependence of the cytotoxicity and metabolism of these compounds. The parent compound 5NQ, its 5NQ, its 8-methyl analogue (8Me5NQ) and the 8-methylamino analogue (8NHMe-5NQ) each showed similar hypoxic selectivity (ratio of concentration x time for 90% kill for zero versus 20% oxygen of 13-18-, 30-69- and 1.2-1.4-fold respectively in the three cell lines tested (AA8 Chinese hamster ovary, EMT6/Ak mouse mammary tumour and FME human melanoma). The cytotoxicity and metabolism (covalent binding) of radiolabelled 8Me-5NQ was investigated in AA8 cultures over a range of oxygen tensions (0-95%). The oxygen tension in solution required for 50% inhibition of log cell kill or adduct formation observed under anoxia (C50) was 0.01 and 0.02% oxygen respectively, suggesting that bioreductive alkylation is the mechanism of 8Me-5NQ toxicity. The K-value (oxygen concentration for cytotoxic potency equal to the mean of the potencies at zero and infinite oxygen) was similar (0.02% oxygen). Calculations based on measured rate constants for formation of the nitroradical anion of 8Me-5NQ and rates of radical loss through disproportionation or reaction with oxygen, predict a K-value for 8Me-5NQ of 0.025% oxygen, in good agreement with the experimentally determined value. Modelling of cell killing expected by the combination of 8Me-5NQ plus radiation suggested that tumour cells at intermediate oxygen tensions (0.01-1%) will be partially resistant to this treatment, and would limit the use of these 5-nitroquinolines in combination with radiation, unless sufficient drug could be delivered to cause extensive killing in the anoxic compartment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T., HOWARD-FLANDERS P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature. 1956 Nov 3;178(4540):978–979. doi: 10.1038/178978a0. [DOI] [PubMed] [Google Scholar]
- Biaglow J. E., Durand R. E. The effects of nitrobenzene derivatives on oxygen utilization and radiation response of an in vitro tumor model. Radiat Res. 1976 Mar;65(3):529–539. [PubMed] [Google Scholar]
- Biaglow J. E., Durand R. E. The enhanced radiation response of an in vitro tumour model by cyanide released from hydrolysed amygdalin. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Apr;33(4):397–401. doi: 10.1080/09553007814550311. [DOI] [PubMed] [Google Scholar]
- Brown J. M. Targeting bioreductive drugs to tumours: is it necessary to manipulate blood flow? Int J Radiat Biol. 1991 Jul-Aug;60(1-2):231–236. doi: 10.1080/09553009114551911. [DOI] [PubMed] [Google Scholar]
- Chapman J. D., Baer K., Lee J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res. 1983 Apr;43(4):1523–1528. [PubMed] [Google Scholar]
- Chapman J. D., Dugle D. L., Reuvers A. P., Meeker B. E., Borsa J. Letter: Studies on the radiosensitizing effect of oxygen in Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Oct;26(4):383–389. doi: 10.1080/09553007414551361. [DOI] [PubMed] [Google Scholar]
- Chapman J. D. Measurement of tumor hypoxia by invasive and non-invasive procedures: a review of recent clinical studies. Radiother Oncol. 1991;20 (Suppl 1):13–19. doi: 10.1016/0167-8140(91)90181-f. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Degn H., Wohlrab H. Measurement of steady-state values of respiration rate and oxidation levels of respiratory pigments at low oxygen tensions. A new technique. Biochim Biophys Acta. 1971 Sep 7;245(2):347–355. doi: 10.1016/0005-2728(71)90153-8. [DOI] [PubMed] [Google Scholar]
- Denny W. A., Atwell G. J., Roberts P. B., Anderson R. F., Boyd M., Lock C. J., Wilson W. R. Hypoxia-selective antitumor agents. 6. 4-(Alkylamino)nitroquinolines: a new class of hypoxia-selective cytotoxins. J Med Chem. 1992 Dec 25;35(26):4832–4841. doi: 10.1021/jm00104a008. [DOI] [PubMed] [Google Scholar]
- Dulhanty A. M., Whitmore G. F. Chinese hamster ovary cell lines resistant to mitomycin C under aerobic but not hypoxic conditions are deficient in DT-diaphorase. Cancer Res. 1991 Apr 1;51(7):1860–1865. [PubMed] [Google Scholar]
- Durand R. E. Adriamycin: a possible indirect radiosensitizer of hypoxic tumor cells. Radiology. 1976 Apr;119(1):217–222. doi: 10.1148/119.1.217. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Chemosensitivity testing in V79 spheroids: drug delivery and cellular microenvironment. J Natl Cancer Inst. 1986 Jul;77(1):247–252. [PubMed] [Google Scholar]
- FROESE G. The respiration of ascites tumour cells at low oxygen concentrations. Biochim Biophys Acta. 1962 Mar 12;57:509–519. doi: 10.1016/0006-3002(62)91158-7. [DOI] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985 Sep;11(9):1699–1707. doi: 10.1016/0360-3016(85)90223-8. [DOI] [PubMed] [Google Scholar]
- Hoban P. R., Walton M. I., Robson C. N., Godden J., Stratford I. J., Workman P., Harris A. L., Hickson I. D. Decreased NADPH:cytochrome P-450 reductase activity and impaired drug activation in a mammalian cell line resistant to mitomycin C under aerobic but not hypoxic conditions. Cancer Res. 1990 Aug 1;50(15):4692–4697. [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Koch C. J., Skov K. A. Comparisons of cellular radiation response using absolute rather than relative parameters. Radiat Res. 1992 Oct;132(1):40–49. [PubMed] [Google Scholar]
- Koch C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993 Sep 1;53(17):3992–3997. [PubMed] [Google Scholar]
- Liu Y. Y., Lu A. Y., Stearns R. A., Chiu S. H. In vivo covalent binding of [14C]trinitrotoluene to proteins in the rat. Chem Biol Interact. 1992 Mar;82(1):1–19. doi: 10.1016/0009-2797(92)90010-i. [DOI] [PubMed] [Google Scholar]
- Marshall R. S., Koch C. J., Rauth A. M. Measurement of low levels of oxygen and their effect on respiration in cell suspensions maintained in an open system. Radiat Res. 1986 Oct;108(1):91–101. [PubMed] [Google Scholar]
- Marshall R. S., Paterson M. C., Rauth A. M. DT-diaphorase activity and mitomycin C sensitivity in non-transformed cell strains derived from members of a cancer-prone family. Carcinogenesis. 1991 Jul;12(7):1175–1180. doi: 10.1093/carcin/12.7.1175. [DOI] [PubMed] [Google Scholar]
- Marshall R. S., Paterson M. C., Rauth A. M. Studies on the mechanism of resistance to mitomycin C and porfiromycin in a human cell strain derived from a cancer-prone individual. Biochem Pharmacol. 1991 May 1;41(9):1351–1360. doi: 10.1016/0006-2952(91)90108-h. [DOI] [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Modification of the cytotoxic activity of mitomycin C by oxygen and ascorbic acid in Chinese hamster ovary cells and a repair-deficient mutant. Cancer Res. 1986 Jun;46(6):2709–2713. [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Oxygen and exposure kinetics as factors influencing the cytotoxicity of porfiromycin, a mitomycin C analogue, in Chinese hamster ovary cells. Cancer Res. 1988 Oct 15;48(20):5655–5659. [PubMed] [Google Scholar]
- Mueller-Klieser W., Schlenger K. H., Walenta S., Gross M., Karbach U., Hoeckel M., Vaupel P. Pathophysiological approaches to identifying tumor hypoxia in patients. Radiother Oncol. 1991;20 (Suppl 1):21–28. doi: 10.1016/0167-8140(91)90182-g. [DOI] [PubMed] [Google Scholar]
- Mulcahy R. T. Effect of oxygen on misonidazole chemosensitization and cytotoxicity in vitro. Cancer Res. 1984 Oct;44(10):4409–4413. [PubMed] [Google Scholar]
- O'Brien P. J., Kaul H. K., Rauth A. M. Differential cytotoxicity of diaziquone toward Chinese hamster ovary cells under hypoxic and aerobic exposure conditions. Cancer Res. 1990 Mar 1;50(5):1516–1520. [PubMed] [Google Scholar]
- Robertson N., Stratford I. J., Houlbrook S., Carmichael J., Adams G. E. The sensitivity of human tumour cells to quinone bioreductive drugs: what role for DT-diaphorase? Biochem Pharmacol. 1992 Aug 4;44(3):409–412. doi: 10.1016/0006-2952(92)90429-m. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Y. C., Rauth A. M. Oxygen tension, cellular respiration, and redox state as variables influencing the cytotoxicity of the radiosensitizer misonidazole. Radiat Res. 1982 Jul;91(1):104–123. [PubMed] [Google Scholar]
- Vanderkooi J. M., Erecińska M., Silver I. A. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol. 1991 Jun;260(6 Pt 1):C1131–C1150. doi: 10.1152/ajpcell.1991.260.6.C1131. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- Whillans D. W., Hunt J. W. A rapid-mixing comparison of the mechanisms of radiosensitization by oxygen and misonidazole in CHO cells. Radiat Res. 1982 Apr;90(1):126–141. [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Wilson W. R., Moselen J. W., Cliffe S., Denny W. A., Ware D. C. Exploiting tumor hypoxia through bioreductive release of diffusible cytotoxins: the cobalt(III)-nitrogen mustard complex SN 24771. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):323–327. doi: 10.1016/0360-3016(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Siim B. G., Denny W. A., van Zijl P. L., Taylor M. L., Chambers D. M., Roberts P. B. 5-Nitro-4-(N,N-dimethylaminopropylamino)quinoline (5-nitraquine), a new DNA-affinic hypoxic cell radiosensitizer and bioreductive agent: comparison with nitracrine. Radiat Res. 1992 Sep;131(3):257–265. [PubMed] [Google Scholar]


