Abstract
Adhesion of parasite-infected red blood cells to the vascular endothelium is a critical event in the pathogenesis of malaria caused by Plasmodium falciparum. Adherence is mediated by the variant erythrocyte membrane protein 1 (PfEMP1). Another protein, erythrocyte membrane protein-3 (PfEMP3), is deposited under the membrane of the parasite-infected erythrocyte but its function is unknown. Here we show that mutation of PfEMP3 disrupts transfer of PfEMP1 to the outside of the P.falciparum-infected cell. Truncation of the C-terminal end of PfEMP3 by transfection prevents distribution of this large (>300 kDa) protein around the membrane but does not disrupt trafficking of the protein from the parasite to the cytoplasmic face of the erythrocyte membrane. The truncated PfEMP3 accumulates in structures that appear to be associated with the erythrocyte membrane. We show that accumulation of mutated PfEMP3 blocks the transfer of PfEMP1 onto the outside of the parasitized cell surface and suggest that these proteins traffic through an erythrocyte membrane-associated compartment that is involved in the transfer of PfEMP1 to the surface of the parasite-infected red blood cell.
Keywords: cytoadherence/malaria/PfEMP1/PfEMP3/protein trafficking
Introduction
Plasmodium falciparum causes the most severe form of malaria in humans and is responsible for >2 million deaths per year. A major complication in human infection is cerebral malaria that causes many of the deaths associated with this disease. The ability of P.falciparum-infected red cells to sequester in the deep vascular beds of the brain is believed to be an important factor in the development of cerebral malaria (MacPherson et al., 1985). During development of the intracellular asexual form of the parasite, extensive remodelling of the human red blood cell occurs that alters both the structural and functional properties of the infected red cell, including the ability of the cell to adhere to the vascular endothelium (Deitsch and Wellems, 1996).
Cytoadherence of P.falciparum-infected erythrocytes has been strongly associated with expression of the parasite-encoded erythrocyte membrane protein 1 (PfEMP1), a high molecular weight, variable protein encoded by members of the var gene family (Baruch et al., 1995; Smith et al., 1995; Su et al., 1995). PfEMP1 is transferred onto the erythrocyte membrane with the N-terminal (binding) region located on the external surface of the infected red blood cell and the C-terminal domain on the cytoplasmic face (Baruch et al., 1995; Smith et al., 1995). PfEMP1 confers the ability of the cell to bind to receptors including CD36 (Baruch et al., 1997), intercellular cell adhesion molecule 1 (ICAM-1) (Baruch et al., 1996) and chondroitin sulfate A (Reeder et al., 1999). Switches in var gene expression result in production of different PfEMP1 proteins that are believed to be responsible for changes in the observed binding phenotype (Smith et al., 1995). PfEMP1 appears to be concentrated on the exterior surface of knobs (Baruch et al., 1995), small electron-dense structures that protrude from the infected red cell surface and contain the knob-associated histidine-rich protein (KAHRP) (Culvenor et al., 1987). Disruption of the KAHRP gene has shown that this protein is essential for knob formation (Crabb et al., 1997a).
Previous work has shown that some P.falciparum strains lose the capacity to cytoadhere after prolonged periods of in vitro culture. Loss of adherence occurs concomitantly with deletion of up to 130 kb of a subtelomeric region of chromosome 2 (Corcoran et al., 1986). The deleted region contains 19 open reading frames (Gardner et al., 1998) any of which potentially are responsible for the loss of cytoadherence. The break-point of multiple chromosome deletion events occurs in the KAHRP gene (Pologe et al., 1987). On the telomeric side of KAHRP is the gene encoding P.falciparum erythrocyte membrane protein 3 (PfEMP3), a protein associated with the erythrocyte membrane of parasite-infected red cells but of unknown function (Pasloske et al., 1993). The deletion of this region of the chromosome, and loss of cytoadherence, correlates with the absence of knob structures (Culvenor et al., 1987; Pologe et al., 1987).
Here, we describe the targeted mutagenesis and disruption of the PfEMP3 gene by transfection of a P.falciparum parasite line (3D7k+) and show that truncation of PfEMP3 blocks the transfer of PfEMP1 onto the outside of the parasite-infected red blood cell. Moreover, we show that the truncated PfEMP3 accumulates in a structure associated with the red blood cell membrane. We provide evidence that suggests that both PfEMP1 and PfEMP3 traffic through a red blood cell membrane-associated compartment that appears to be responsible for assembly of the cytoadherence complex.
Results
Mutagenesis of the PfEMP3 gene
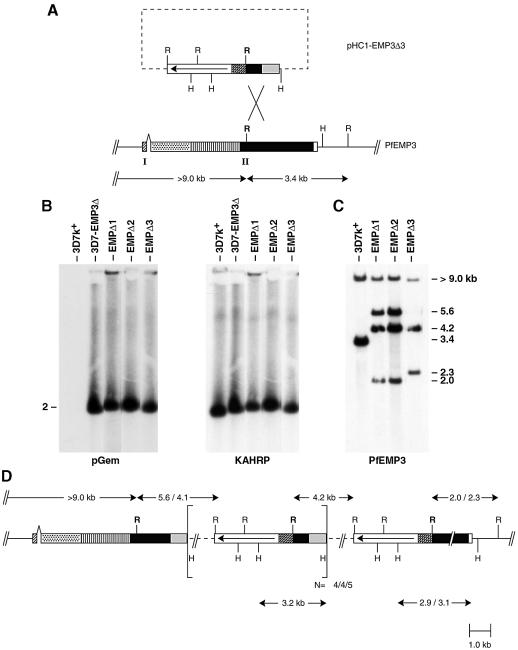
To assess the role of PfEMP3 in cytoadherence and knob formation, stable transfectants of P.falciparum (Crabb and Cowman, 1996; Wu et al., 1996) were created with a defined mutation in the PfEMP3 coding region. A 719 bp segment of the 3′ repeat region of the PfEMP3 gene was inserted into the plasmid transfection vector pHC1 (Crabb et al., 1997b) to derive the vector pHC1-EMP3Δ3 (Figure 1A). The construct was used to transfect 3D7k+. Stable transfectants containing pHC1-EMP3Δ3 as episomes were isolated and selected to obtain parasites with homologous integration. Chromosomes from the parent 3D7k+ and the uncloned transfectant 3D7-EMP3Δ were separated by pulsed-field gel electrophoresis (PFGE) (Chu et al., 1986) and probed with the plasmid (pGem) or the KAHRP gene (Figure 1B). The KAHRP probe hybridized to chromosome 2 in 3D7k+ and the transfected line. The pGem probe showed no hybridization to 3D7k+ but did hybridize to chromosome 2 of 3D7-EMP3Δ. Therefore, the plasmid pHC1-EMP3Δ3 had integrated into chromosome 2.
Fig. 1. PfEMP3Δ3 transfection construct and integration events. (A) The plasmid construct, pHC1-EMP3Δ3, contains an insert of the 3′ repeat region of PfEMP3 exon 2 cloned downstream of the calmodulin promoter region in pHC1. The open arrowed box shows the pyrimethamine selection cassette for the generation of stable transfectants.The cross-hatched and grey boxes indicate the calmodulin 5′ and hsp86 3′ terminator sequences, respectively. A schematic representation of the gene encoding PfEMP3 is shown (Gardner et al., 1998). The gene has a two exon structure designated by I and II. The first exon (cross-hatched) encodes a putative signal sequence followed by a region that is non-repetitive (stippled) and two highly repetitive regions consisting of two sets of repeat regions (vertical hatching and solid filled box). The two repeat regions are followed by a non-repetitive unique region (empty box). R and H represent EcoRI and HincII restriction sites used in restriction site mapping. The cross indicates the region where gene disruption has taken place. (B) PFGE showing chromosomes from parental 3D7k+ and the transfected cloned lines EMPΔ1, EMPΔ2 and EMPΔ3. Identical filters were hybridized with pGem and KAHRP. (C) EcoRI genomic digests and Southern analysis of 3D7k+, EMPΔ1, EMPΔ2 and EMPΔ3 probed with PfEMP3 showing disruption of the endogenous PfEMP3 gene. (D) Map of the integration event for truncation mutants EMPΔ3, EMPΔ2 and EMPΔ1. Numbers refer to the length of designated restriction fragments in kb. All integrants have in common the >9.0 kb endogenous EcoRI band and the 4.2 kb plasmid band. Numbers separated by a backslash represent sizes of restriction fragments for EMPΔ2 and EMPΔ1 versus EMPΔ3, respectively. Square brackets show the plasmid repeat unit arising from multiple copies of the plasmid integrating into the PfEMP3 gene. The number of plasmid copies integrated per clone is shown outside the brackets for EMPΔ3, EMPΔ2 and EMPΔ1, respectively. The PfEMP3 coding region is designated as shown in (A).
To test if the transfected plasmid had integrated into the PfEMP3 gene, genomic DNA from 3D7k+ and three transfected cloned lines EMPΔ1, EMPΔ2 and EMPΔ3, derived from the transfected parental line 3D7-EMP3Δ (see below), were digested with EcoRI prior to Southern blotting. The filters were probed with the PfEMP3 fragment used in the vector pHC1-EMP3Δ3 (Figure 1 C). In 3D7k+, two fragments of >9 and 3.4 kb were observed, whereas in EMPΔ1 the probe hybridized to four fragments of 2.0, 4.2, 5.6 and >9.0 kb, indicating that the plasmid had integrated into the PfEMP3 gene. The cloned line EMPΔ2 gave an identical hybridization pattern to that seen in EMPΔ1, suggesting that they shared the same integration site. Hybridization of the PfEMP3 probe to the EMPΔ3 cloned line gave three fragments of 2.3, 4.2 and >9.0 kb consistent with homologous integration of the transfection plasmid into a different site within the 3′ repeat region of the PfEMP3 gene compared with EMPΔ1 and EMPΔ2.
To confirm the position of each integration event and the number of plasmid copies inserted, the structure of the PfEMP3 gene was determined by restriction mapping using HincII and EcoRI and probing with the single copy probe calmodulin (Figure 1D and data not shown). This showed that six copies of the plasmid were inserted into the 3′ repeat region of the PfEMP3 gene in EMPΔ1, resulting in deletion of 1.4 kb of the 3′ repeats of the gene. Similarly, EMPΔ3 has inserted five copies of the plasmid into the 3′ repeat region and this has resulted in deletion of 1.1 kb of 3′ repeats. The structure of the integration event in the transfectant EMPΔ2 was found to be the same as in EMPΔ1 except that five plasmid copies had been inserted into the gene. These results confirm that pHC1-EMP3Δ3 had integrated into the PfEMP3 gene resulting in truncation of the endogenous gene at the 3′ end. Expression of the PfEMP3 gene from these transfectants should result in an altered protein product that was truncated at the C-terminus by between 370 and 470 amino acids.
Altered PfEMP3 was expressed in the P.falciparum transfectants
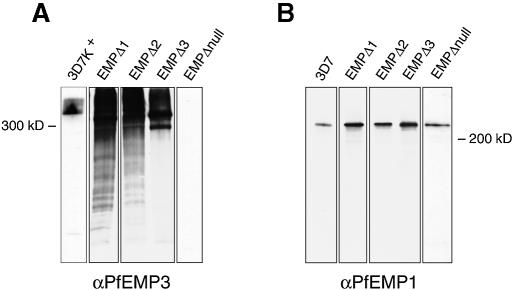
Proteins from trophozoite-stage parasites of 3D7k+, EMPΔ1, EMPΔ2 and EMPΔ3 were extracted and examined by immunoblot analysis to determine if expression of PfEMP3 was altered in the transfectants. Filters were probed with anti-PfEMP3 antiserum and a protein band of >300 kDa was detected in 3D7k+ (Figure 2A). A very different pattern was observed for the transfectants EMPΔ1, EMPΔ2 and EMPΔ3, with a major band smaller than that seen in 3D7k+ and a smear evident in each lane suggesting overexpression and degradation of the protein. Identical protein extracts were probed with antibodies to the conserved acidic terminal segment (ATS) of PfEMP1 (Crabb et al., 1997a) and a single protein band of >200 kDa was seen in 3D7k+ and the transfected parasites (Figure 2B). Therefore, expression of PfEMP1 has not been altered in the transfectants.
Fig. 2. Altered PfEMP3 expression in the transfected parasite lines. Triton X-100-insoluble/SDS soluble fractions were separated by SDS–PAGE and blotted to PVDF membrane. Identical membranes were probed with antibodies to either (A) PfEMP3 or (B) the ATS portion of PfEMP1.
To rule out the possibility that the transfectants had switched to the transcription of a new var gene, we performed RT–PCR. PCR products were generated using degenerate oligonucleotides for both the Duffy binding-like (DBL) region 1 as well as across the intron between the sequence of variable length (SVL) and the ATS (Crabb et al., 1997a). A dominant var gene transcript was detected in 3D7k+, EMPΔ1, EMPΔ2 and EMPΔ3. This result was consistent with the detection of a single protein band of equal size in 3D7k+ and the different transfectants using anti-ATS antibodies. These data provide evidence that the same var gene was expressed and no switch event to another PfEMP1 protein had occurred.
Altered expression of PfEMP3 affects adherence to CD36
To determine if altered expression of PfEMP3 had affected adherence to different receptors, we quantified adhesion of parasitized cells to purified receptors using static assays (Crabb et al., 1997a). Of six clones analysed initially, only two showed significant binding to CD36 (EMPΔ2 and EMPΔ3) although this was greatly reduced compared with the parental line 3D7k+. Three clones that represented the different phenotypes observed (EMPΔ1, EMPΔ2 and EMPΔ3) were analysed further. The structure of the integrated plasmids for each clone and expression of altered PfEMP3 is described above.
In static adhesion assays, the 3D7k+ parental line adhered to purified CD36 as expected (Table I) (Crabb et al., 1997a). In contrast, the transfected parasite lines EMPΔ3, EMPΔ2 and EMPΔ1 bound to CD36 at 16, 9 and 0.01% respectively, relative to wild-type 3D7-infected red blood cells. All lines were tested for ICAM-1 and chondroitin sulfate A adherence but showed no significant binding to these receptors (data not shown). In a separate experiment, binding to CD36 was shown to be sensitive to trypsin cleavage (Table I) and all adherence was ablated with 10 µg/ml of trypsin. Co-incubation of the cells with trypsin and soybean trypsin inhibitor (STI) did not affect adhesion to CD36. The sensitivity to trypsin of CD36 adhesion of 3D7k+ and the transfected lines is typical of PfEMP1-mediated binding to CD36 (Leech et al., 1984).
Table I. Adherence and trypsin sensitivity of P.falciparum to CD36a.
| Parasite clone | Trypsin concentration (µg/ml) |
Soybean trypsin inhibitor | |||
|---|---|---|---|---|---|
| 0 | 1 | 10 | 100 | ||
| 3D7k+ | 719 ± 81 | 41 ± 15 | 21 ± 22 | 5 ± 5 | 281 ± 71 |
| 3D7-EMPΔ | 66 ± 23 | ND | ND | ND | ND |
| EMPΔ1 | 3 ± 25 | 4 ± 2 | 2 ± 1 | 0.5 ± 1 | 6 ± 4 |
| EMPΔ2 | 63 ± 39 | ND | ND | ND | ND |
| EMPΔ3 | 115 ± 25 | 35 ± 20 | 5 ± 3 | 3 ± 4 | 129 ± 87 |
aThe results are expressed as infected red blood cells bound per mm2 and show the mean ± SEM assayed in triplicate.
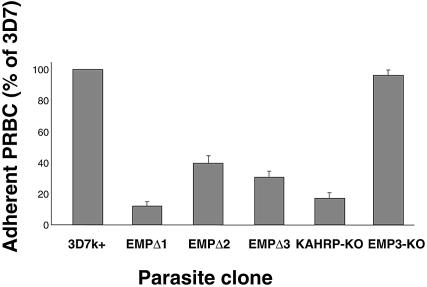
The 3D7k+ parental line and transfectants were tested for the ability to adhere to CD36 under flow conditions that mimic those found in the microvasculature compartment where P.falciparum is known to sequester (Cooke et al., 1994). At a wall shear stress of 0.1 Pa, the transfected parasite lines adhered to CD36 at reduced levels when compared with 3D7k+ (Figure 3). This is in contrast to the KAHRP knockout parasite line, which retained its ability to adhere to CD36 in static assays, but showed markedly reduced binding when tested under flow (Figure 3) (Crabb et al., 1997a).
Fig. 3. Adhesion of 3D7k+, EMPΔ1, EMPΔ2, EMPΔ3, KAHRP knockout lines and EMP null lines to purified, immobilized CD36 under physiologically relevant flow conditions. The number of flowing parasitized red blood cells that adhered to CD36 in the flow chamber at wall shear stress of 0.1 Pa are compared for the parental 3D7k+ and each of the clones. Each of the parasite clones is expressed as a percentage of the parental line 3D7k+. Error bars represent the SEM for three separate experiments on each clone.
Developmental expression of PfEMP1, KAHRP and PfEMP3
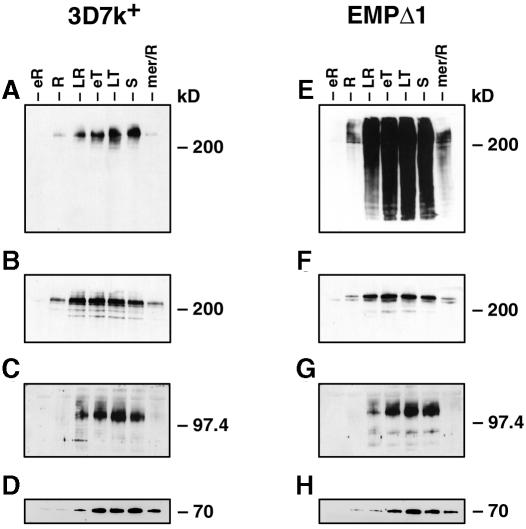
To determine if integration and altered PfEMP3 expression in the transfectants had affected the developmental expression of PfEMP1 and KAHRP in the parasite’s asexual life cycle, samples from tightly synchronized cultures were taken every 8 h, and protein extracts were made and analysed by immunoblot using antibodies to PfEMP3 (Figure 4A and E), PfEMP1 (Figure 4B and F) and KAHRP (Figure 4C and G). Expression of PfEMP3 in 3D7k+ begins from mid to late rings, with trophozoite stages showing maximal amounts of protein. EMPΔ1 parasites showed a very similar timing of expression for PfEMP3; however, there is a large smear throughout development as discussed earlier. Unlike PfEMP3, PfEMP1 expression begins in early ring stages in both 3D7k+ and EMPΔ1, indicating that PfEMP1 production occurs earlier than that of PfEMP3. KAHRP expression occurs at approximately the same time as PfEMP3 in both 3D7k+ and EMPΔ1. These results show that the altered PfEMP3 in the transfected parasite lines does not affect the timing of expression of either PfEMP1 or KAHRP and that PfEMP1 expression occurs before PfEMP3 and KAHRP.
Fig. 4. Developmental expression of PfEMP3, PfEMP1 and KAHRP in 3D7k+ and EMPΔ1. Synchronized ring stages of each parasite line were sampled every 8 h and Triton X-100-insoluble/SDS-soluble fractions analysed by SDS–PAGE. The same extract from 3D7k+ was probed with (A) anti-PfEMP3, (B) anti-ATS, (C) anti-KAHRP and (D) anti-Hsp70 antibodies. Extracts from EMPΔ1 were probed with (E) anti-PfEMP3, (F) anti-ATS, (G) anti-KAHRP and (H) anti-Hsp70 antibodies.
Altered expression of PfEMP3 blocks transfer of PfEMP1 to the red blood cell surface
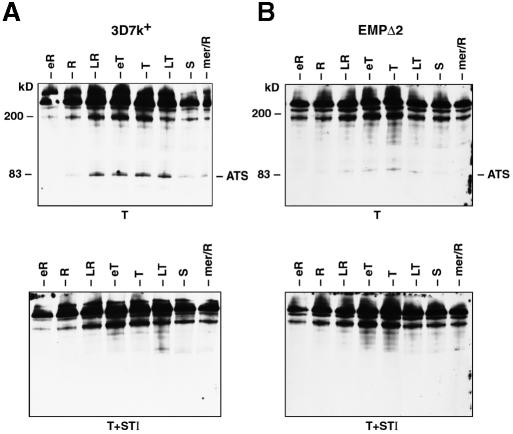
Decreased levels of CD36 adherence in transfectants could be due to lower levels of PfEMP1 on the surface of the infected red cell. To examine this possibility, we developed an assay that allowed us to visualize PfEMP1 exposed on the surface by virtue of its sensitivity to trypsin cleavage. First, the timing of expression of PfEMP1 on the outside surface of the red cell was determined for both 3D7k+ (Figure 5A) and the transfectant EMPΔ2 (Figure 5B), which showed a low level of CD36 adherence (9%). Synchronized cultures were sampled at 4 h intervals and treated either with trypsin or trypsin plus STI followed by extraction of proteins for immunoblot analysis. PfEMP1 protein was detected using an antibody to the cytoplasmic ATS region (Crabb et al., 1997a). The 3D7k+ parental line shows a major protein band of >200 kDa expected for uncleaved PfEMP1 protein (Figure 5A) (Crabb et al., 1997a). A trypsin cleavage product of 83 kDa representing the protected intracellular domain of PfEMP1 is detected with the anti-ATS antiserum starting in ring stages, continuing through to late trophozoites. The control experiment with trypsin and STI gave an identical pattern of bands except for the absence of the 83 kDa product. Therefore, the 83 kDa band in trypsin-treated cells must result from cleavage of PfEMP1 exposed on the surface of the infected red cell. This shows that PfEMP1 is expressed and accumulates before it is exposed on the outside surface of the infected red cell in late rings. Identical experiments were performed with the transfected cell line EMPΔ2 and similar results were obtained, except that the 83 kDa product was present at much lower levels (Figure 5B). This suggested that the low level of binding to CD36 for this parasite line was due to decreased levels of PfEMP1 expressed on the infected red cell surface throughout asexual development.
Fig. 5. Developmental expression of PfEMP1 on the surface of red blood cells infected with 3D7k+ and EMPΔ2. Synchronized ring stages of 3D7k+ and EMPΔ2 were sampled every 4 h and treated with trypsin or trypsin plus STI, and extracts analysed by SDS–PAGE and immunoblot with anti-ATS antibodies to detect cleaved PfEMP1. (A) Upper panel, 3D7k+ treated with trypsin; lower panel, 3D7k+ treated with trypsin plus STI. (B) Upper panel, EMPΔ2 treated with trypsin; lower panel, EMPΔ2 treated with trypsin plus STI.
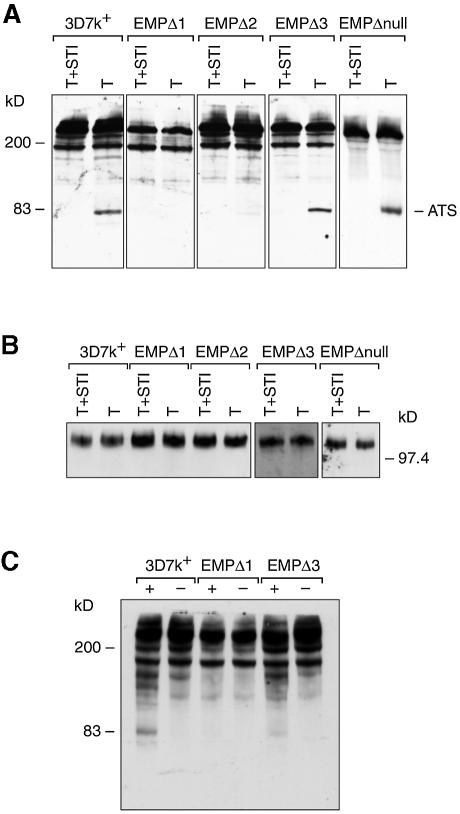
To compare the amount of PfEMP1 exposed on the infected red cell surface between the parental line 3D7k+ and the three transfected lines, trophozoites alone were treated as described above (Figure 6A). In the 3D7k+ line, the expected trypsin cleavage product was observed, whereas in EMPΔ1, which shows little adherence to CD36 (0.01%), no trypsin cleavage product is evident, suggesting that there is no detectable expression of PfEMP1 on the outside surface of the infected red blood cell. Both EMPΔ2 and EMPΔ3 do express some PfEMP1 on the red cell surface and the amount detected is consistent with the lower levels of CD36 adherence observed. In repeat experiments, EMPΔ3 consistently showed a lower amount of cleavage product relative to 3D7k+. These results suggest that although the parental line 3D7k+ and the different transfected lines express equivalent amounts of PfEMP1 protein, there is a decrease in the ability to transfer the protein onto the red cell surface.
Fig. 6. Determination of PfEMP1 exposed on the surface of red blood cells infected with 3D7k+, EMPΔ1, EMPΔ2 and EMPΔ3 by assaying for trypsin and chymotrypsin cleavage. Intact trophozoites of each parasite line were treated with (A) trypsin or trypsin plus STI, (B) trypsin or trypsin plus STI and (C) chymotrypsin (+) or no added chymotrypsin (–), and extracts were analysed by SDS–PAGE and immunoblot with anti-ATS antibodies for (A) and (C) and anti-KAHRP antibodies for (B).
The inability to digest the majority of PfEMP1 with trypsin suggested that only a small proportion of the total expressed protein was located on the red cell surface. It was, however, possible that the undigested PfEMP1 detected was exposed to the outside but was trypsin resistant and did not bind CD36. In order to rule out this possibility, we performed a similar experiment using chymotrypsin (Figure 6C). A very similar pattern was seen for 3D7k+ with and without chymotrypsin except for the appearance of an ∼83 kDa product when the protease was present. No 83 kDa band was detected for the transfectant EMPΔ1, showing that no PfEMP1 is exposed on the outside surface. In contrast, a small amount of the 83 kDa protein was evident for EMPΔ3. These results are consistent with the level of CD36 adherence seen with each of the lines. It also confirms that most of the PfEMP1 protein does not reach the outside of the infected red cell surface.
To rule out the possibility that trypsin had leaked through the erythrocyte membrane to the pool of cytoplasmic PfEMP1, we assessed the integrity of the KAHRP protein located under the infected red cell erythrocyte membrane for both trypsin-treated cells compared with control (STI plus trypsin) samples. A duplicate filter of that shown in Figure 6 was probed with KAHRP antisera. A single KAHRP band was detected in trypsin-treated and control cells (Figure 6 B), indicating that the PfEMP1 cleavage was specific to proteins exposed on the red cell surface.
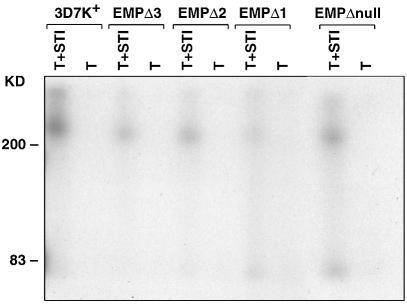
To ensure that the trypsin and chymotrypsin PfEMP1 cleavage product detected by anti-ATS antisera was an accurate measure of erythrocyte surface-located protein, we used the method of surface radio-iodination to confirm the results. Equal numbers of synchronized trophozoites from 3D7k+, EMPΔ1, EMPΔ2 and EMPΔ3 parasites were surface labelled with 125I and the SDS-solubilized pellet was used for immunoprecipitation with the anti-ATS antibodies (Figure 7). A trypsin-sensitive protein band of >200 kDa was detected in 3D7k+ which is typical of the properties of PfEMP1 that have been described previously. The same trypsin-sensitive protein band could be detected in EMPΔ3, EMPΔ2 and EMPΔ1; however, the amount detected was decreased in all three parasite transfectants, with the lowest level detectable in EMPΔ1. This was consistent with the results obtained using both trypsin and chymotrypsin cleavage and confirms that PfEMP1 transfer to the outside of the erythrocyte membrane is reduced in these transfectants.
Fig. 7. Detection of PfEMP1 on the surface of infected erythrocytes by iodination and immunoprecipitation. Live 3D7k+, EMPΔ3, EMPΔ2, EMPΔ1 and the knockout parasite lines were surface labelled with 125I, SDS extracts made and labelled PfEMP1 immunoprecipitated with anti-ATS antisera. Before solubilization, the intact labelled cells were treated with either trypsin plus STI (T+STI) or trypsin alone (T) as described previously (Baruch et al., 1996).
PfEMP3 is not distributed around the underside of the P.falciparum-infected red blood cell in the transfectants
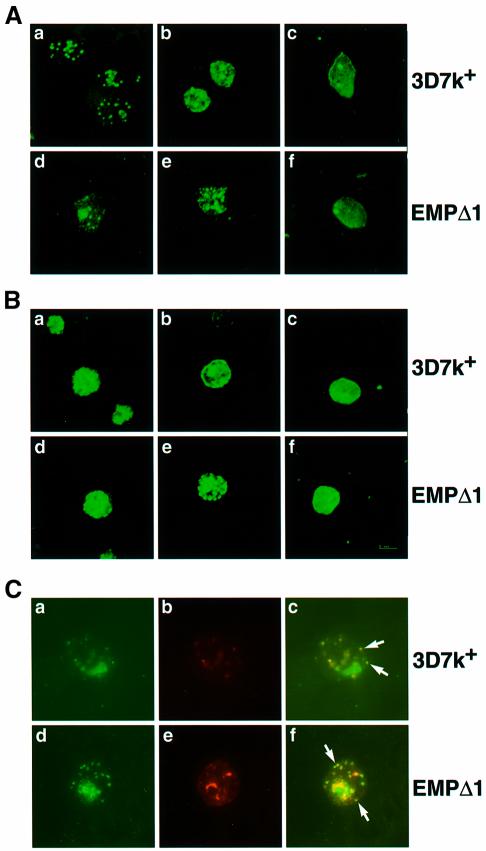
To examine the effect of truncating PfEMP3 on its distribution and also that of PfEMP1 and KAHRP, we compared the transfectant EMPΔ1 with the 3D7k+ parental clone by immunofluorescence using specific affinity-purified antisera to each protein (Figure 8). To determine whether the pattern of expression had altered between parental 3D7k+ and EMPΔ1, smears were taken every 8 h throughout the parasite asexual life cycle. Expression of PfEMP1 in ring stages of the 3D7k+ parental line showed a distinct punctate pattern that was very similar in the transfectant EMPΔ1 (Figure 8A). Late-stage parasites for both 3D7k+ and EMPΔ1 showed similar patterns, with some apparent surface fluorescence in 3D7k+, although there was a large proportion of the protein that appeared to be internal to the membrane (Figure 8B). Although the EMPΔ1 transfectant had a similar pattern, there were some concentrations of PfEMP1 in a punctate pattern, around the underside of the infected red cell membrane.
Fig. 8. Immunolocalization of PfEMP1, PfEMP3 and KAHRP in 3D7k+ and EMPΔ1 mutant parasites. Affinity-purified antibodies to PfEMP1 (anti-ATS), PfEMP3 or KAHRP were reacted to smears of each cloned line. Following incubation with FITC-labelled goat anti-rabbit antibody, cells were visualized by laser confocal microscopy. (A) The P.falciparum cloned lines are as follows: ring stages 3D7k+ labelled with (a) anti-ATS, (b) anti-PfEMP3 and (c) anti-KAHRP; ring stages EMPΔ1 labelled with (d) anti-ATS, (e) anti-PfEMP3 and (f) anti-KAHRP. (B) Mature trophozoites 3D7k+ labelled with (a) anti-ATS, (b) anti-PfEMP3 and (c) anti-KAHRP; mature trophozoites EMPΔ3 labelled with (d) anti-ATS (e) anti-PfEMP3 (f) anti-KAHRP. (C) Co-localization of PfEMP1 and PfEMP3 for both 3D7k+ and EMPΔ1 early trophozoites. The top panel shows 3D7k+ probed with anti-ATS (a), anti-PfEMP3 (b) and dual localization (c). The bottom panel shows EMPΔ1 probed with anti-ATS (d), anti-PfEMP3 (e) and dual localization (f). Arrows indicate examples of vesicle-like structures that contain both PfEMP1 and PfEMP3.
KAHRP antibodies showed similar results for both 3D7k+ and EMPΔ1, with light fluorescence in ring stages (Figure 8A) and strong reactivity in late stages with clear surface fluorescence (Figure 8B). This is consistent with localization of KAHRP under the infected red cell membrane in the knobs (Culvenor et al., 1987; Pologe et al., 1987). These results show that truncation of PfEMP3 has no effect on the localization of the KAHRP protein.
PfEMP3 antibodies again gave similar patterns in ring stages for both 3D7 and EMPΔ1, with some punctate pattern evident (Figure 8A). In late stages, the patterns were very different, with 3D7k+ showing typical surface fluorescence consistent with localization on the undersurface of the infected red cell (Figure 8B). The transfectant EMPΔ1 showed a strong punctate pattern with a concentration of spots apparently aligned directly under the erythrocyte membrane. This suggests that the truncated PfEMP3 can be trafficked out of the parasite to the red cell membrane but does not spread beneath the membrane.
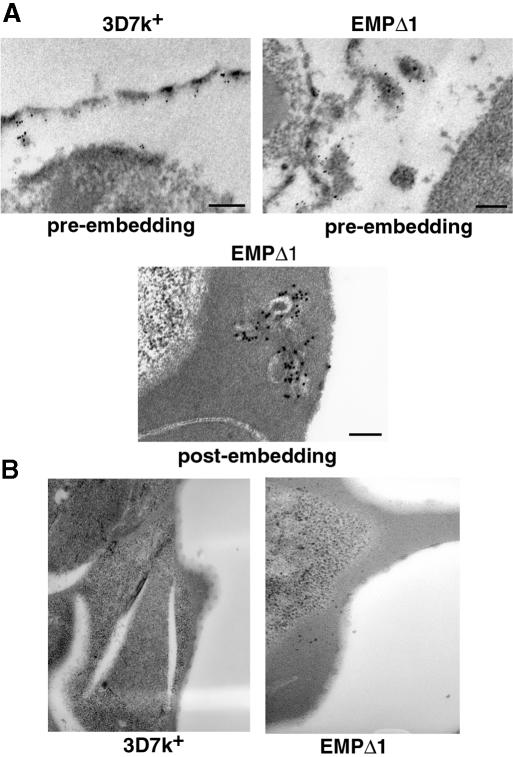
To determine the differences in localization of the truncated PfEMP3 between the transfected parasites and the 3D7k+ parental line, we used immunoelectron microscopy of trophozoite stages and affinity-purified antibodies to PfEMP3. The 3D7k+ and EMPΔ1 parasites were permeabilized and incubated with PfEMP3 antibodies for pre-embedding labelling such that most of the red cell cytoplasm was lost. Localization was visualized with protein A and 5 nM gold particles. In 3D7k+, labelling was distributed along the full length under the cytoplasmic face of the membrane (Figure 9A). In EMPΔ1, no labelling of PfEMP3 could be seen closely associated with the underside of the red cell membrane, confirming the immunofluorescence experiments and suggesting that PfEMP3 had not been distributed around the cytoplasmic face of the red blood cell membrane. Interestingly, there were membranous structures attached to the red cell membrane that strongly labelled with anti-PfEMP3 antibodies, suggesting that the truncated PfEMP3 protein had accumulated in these structures. These structures were regularly spaced around the membrane of the red blood cell and may represent functional units for the assembly and transfer of PfEMP1 across the erythrocyte membrane.
Fig. 9. Immunoelectron microscopy of PfEMP3 in 3D7k+ and EMPΔ1 mutant. (A) Pre- and post-embedding labelling immunolocalization of PfEMP3 in trophozoites. Scale bar represents 200 nm. (B) Transmission electron microscopy of 3D7k+ and EMPΔ1 parasite lines showing knobs.
The transfected line EMPΔ1 was incubated with anti-PfEMP3 antibodies in post-embedding labelling experiments and visualized using electron microscopy. Accumulation of PfEMP3 could be seen in membranous structures, some of which appeared to be associated with the red cell membrane. Taken together, the immunoelectron microscopy and immunofluorescence experiments with the transfectant EMPΔ1 have shown that truncated PfEMP3 accumulates in structures associated with, and regularly spaced around, the underside of the red cell membrane.
If the structures visualized by antibodies to PfEMP3 in EMPΔ1 are involved in trafficking and transfer of PfEMP1 across the red blood cell membrane, it would be expected that both PfEMP1 and PfEMP3 should co-localize within them. To test this, we performed immunofluorescence using antibodies to both PfEMP1 and PfEMP3 in co-localization experiments throughout the asexual life cycle for both 3D7k+ and EMPΔ1. PfEMP1 was expressed before PfEMP3 and initially no co-localization was observed (data not shown). Co-localization of PfEMP1 and PfEMP3 was seen in late rings for both 3D7k+ and the transfected line EMPΔ1 in vesicle-like structures that were closely positioned near the red cell membrane (Figure 8C). In 3D7k+, these structures were observed transiently. As the 3D7k+ parasite developed, PfEMP3 became more evenly spread around the under surface of the red cell membrane. In contrast, with EMPΔ1, the vesicle-like structures became larger within the cytoplasm of the infected red cell. These observations support our contention that the structures observed are involved in the trafficking and transfer of PfEMP1 across the infected red cell membrane.
Previously, it has been shown that the KAHRP protein is essential for knob formation and that these structures are required for cytoadherence under physiologically relevant flow conditions (Crabb et al., 1997a). PfEMP3 also associates with knobs (Pasloske et al., 1993), so it was of interest to determine whether truncation of this protein affected the formation of knobs. Mature trophozoites of 3D7k+ and EMPΔ1 were examined by electron microscopy and both lines showed the presence of numerous, typical, electron-dense knobs (Figure 9B). Truncation of PfEMP3, therefore, does not appear to have affected the formation of knobs.
PfEMP3 null parasite-infected red blood cells bind to CD36
There were two possible explanations for the decrease in transfer of PfEMP1 to the outside surface of the infected red cell of EMPΔ1, EMPΔ2 and EMPΔ3 transfectants. First, PfEMP3 may play a role in transfer of PfEMP1, thus exposing the adherence domains of this protein on the outer surface of the infected red blood cell. Secondly, it was possible that the physical accumulation of the truncated form of PfEMP3 in the structures we have identified under the infected red blood cell membrane had blocked the ability to transfer PfEMP1 to the surface. In order to differentiate between these two possibilities, we disrupted the PfEMP3 gene in 3D7k+ by targeting integration of a transfection plasmid at the 5′ end of the gene. This was achieved by inserting a 1.0 kb fragment encoding the 5′ unique region of PfEMP3 (Gardner et al., 1998) into the transfection plasmid pHC1 (Crabb et al., 1997b) to obtain the plasmid pHC1EMP3Δ5U. The plasmid was transfected into 3D7k+ to derive the transfected line EMPΔnull. The transfection plasmid was shown to have integrated into the PfEMP3 gene by both PFGE and Southern blot analysis (data not shown). Immunoblot analysis of these parasites with anti-PfEMP3 antibodies showed that these parasites did not express the PfEMP3 protein but expressed normal levels of PfEMP1 (Figure 2), which was in agreement with the structure of the plasmid integration into the PfEMP3 gene. The EMPΔnull transfectant was able to form knob structures as shown by electron microscopy (data not shown).
Static and flow adherence assays were carried out on EMPΔnull-infected red cells compared with the 3D7k+ parental cloned line. Adherence was measured at 76 ± 11% for static assays and 95% in flow assays (Figure 3), respectively, on purified CD36 receptor, indicating a slight reduction in binding relative to 3D7k+-infected red cells (Figure 3). In order to determine the amount of surface-exposed PfEMP1 on the infected red cell, we used the trypsin cleavage assay as described above. EMPΔnull parasites showed the same relative levels of PfEMP1 as 3D7k+ controls (Figure 6), consistent with the ability of both to adhere efficiently to CD36. This was confirmed using surface radio-iodination and immunoprecipitation with anti-ATS antibodies (Figure 7). Therefore, expression and accumulation of the truncated forms of PfEMP3 in EMPΔ1, EMPΔ2 and EMPΔ3 has physically blocked the transfer of PfEMP1 to the surface of the parasite-infected red blood cell.
Discussion
The red blood cell undergoes a number of changes after infection with P.falciparum producing new structures in the red blood cell cytoplasm and protein complexes at the membrane surface (Deitsch and Wellems, 1996). This results in the ability of the infected red blood cell to bind to receptors on endothelial cells, a property mediated by the protein family PfEMP1 (Baruch et al., 1995, 1997; Smith et al., 1995; Reeder et al., 1999). Previously, it has been shown that KAHRP is an important accessory protein required for adherence of PfEMP1 to CD36 under physiologically relevant flow conditions (Crabb et al., 1997a). These results also suggested that there were other accessory proteins required for PfEMP1-mediated cell adherence. The protein PfEMP3 previously has been localized under the red blood cell membrane of the P.falciparum-infected red blood cell (Pasloske et al., 1993) and we have investigated its role by targeted gene disruption. We have shown that truncation of this protein blocks transfer of PfEMP1 to the outer surface of the infected red blood cell and this has allowed us to identify a membranous compartment that forms transiently under the infected erythrocyte membrane. We suggest that this compartment is responsible for sorting and trafficking of PfEMP1 to the outside surface of the infected erythrocyte.
Truncation of the PfEMP3 protein did not disrupt its trafficking to the red cell membrane; however, it was concentrated in structures that appear to be attached to the membrane. This suggests that mutation of PfEMP3 has prevented the distribution of the protein beneath the red cell membrane surface and it is possible that we have disrupted or deleted a protein-binding domain that is required for correct sorting and trafficking. Accumulation of truncated PfEMP3 in regularly spaced structures, apparently attached to the parasite-infected red cell membrane, suggests that they may be important in trafficking of proteins and/or assembly of a cytoadherence complex. The ability of parasite-infected red blood cells to cytoadhere begins in the late ring stage of the asexual life cycle (Gardner et al., 1996) and, at this time, it was possible to visualize vesicle-like structures that are closely associated with the red cell membrane. Additionally, PfEMP1 co-localizes with PfEMP3 in these structures transiently during the development of the parasite. In wild-type parasites, PfEMP3 quickly spreads around the underside of the red blood cell membrane; however, truncation of PfEMP3 appears to have prevented this process and allowed us to detect the structures associated with the membrane more easily. It is likely that these transient structures are the result of vesicular fusion at specific points under the red cell surface. We suggest that trafficking to and assembly of parasite-encoded proteins on the red cell membrane do not occur randomly around the red cell membrane but at specific structures that are important for assembly of PfEMP1 on the outside surface of the infected red cell.
The observation that there was reduced ability of the transfectants expressing truncated PfEMP3 to transfer PfEMP1 onto the outside of the red cell membrane supports the role of the structures in the transfer of PfEMP1. Our results clearly show that accumulation of truncated PfEMP3 physically blocks transfer of PfEMP1 rather than playing a direct role in this process. The increased levels of expression of truncated PfEMP3 protein in the different cloned transfectants correlate with the decreased ability of the parasitized red cells to adhere to CD36. Additionally, the EMPΔnull transfectant, which expresses no detectable PfEMP3, is able to cytoadhere to CD36, although this was slightly reduced compared with the parental parasite line.
The PfEMP3 protein clearly is not essential for the transfer of PfEMP1 to the outside surface of the parasite-infected red cell. However, it is interesting that PfEMP3 shows 27% identity and 48% similarity to a yeast protein Uso1p over a contiguous 1000 amino acid coiled-coil domain of PfEMP3 (Nakajima et al., 1991). Uso1p acts as a tethering protein that docks vesicles for endoplasmic reticulum (ER) to Golgi transport in yeast (Cao et al., 1998). While it is unlikely that PfEMP3 is the P.falciparum homologue of Uso1p, it may share some functional similarity such as tethering transport vesicles. Blockage of this process presumably would cause a build up of vesicles and create structures similar to that seen in the PfEMP3 mutant parasites. Interestingly, overexpression of a number of proteins can restore ER to Golgi transport in Uso1p loss-of-function mutants (Cao et al., 1998). It is possible that there are a number of proteins in P.falciparum that have similar functions that may compensate for the loss of PfEMP3.
Independent clones derived from the truncated PfEMP3 transfectants revealed that there were at least two independent integration events. Additionally, some of the clones had differing numbers of the transfection plasmid integrated, suggesting that these may also be independent integration events that had occurred in similar positions in the 3′ repeats. Integration of multicopies of the plasmid by homologous recombination has occurred in a number of other transfections in P.falciparum (Wu et al., 1996; Crabb et al., 1997a; Triglia et al., 1998). It was apparent from analysis of six different cloned transfected lines that there was some variation in the ability of these cells to adhere to CD36. The likely explanation for this variation was the different position of the integration events within the PfEMP3 gene and the number of plasmid copies inserted affecting the level of PfEMP3 expression. Expression of truncated PfEMP3 was increased in the three clones analysed; however, this was to differing amounts, suggesting that the integration site differentially affected stability of the protein and/or elements have been removed from the 3′ end of the gene important in regulation of gene expression.
Expression of PfEMP1 begins in early ring stages and appears on the surface of the parasite-infected red cell in late rings, which is consistent with the timing of the ability of these cells to adhere (Gardner et al., 1996). This suggests that PfEMP1 accumulates in the red cell cytoplasm before being transferred to the outside of the membrane surface. We chose to assay the presence of PfEMP1 on the outside surface of the red blood cell membrane using trypsin cleavage to distinguish between surface and cytoplasmic protein. An alternative method was to use surface iodination of proteins and immunoprecipitation of PfEMP1 (Leech et al., 1984); however, it is clear that this method can label some internal proteins such as α- and β-spectrin (Baruch et al., 1996). We ensured that trypsin was not reaching the inside of the parasite-infected red cell by analysing the integrity of the KAHRP protein which is localized just under the red cell membrane. No degradation of this protein was detected in any of the parasite-infected red cells that had been treated with trypsin. Therefore, the 83 kDa protein detected by anti-ATS antibodies after trypsin treatment must represent the cytoplasmic ATS region and include the hydrophobic transmembrane portion as well as a small part of the sequence of variable length (SVL) (Crabb et al., 1997a). The trypsin cleavage site must be in the SVL region and this is consistent with the size of 83 kDa detected by the anti-ATS antibodies. Interestingly, this method allowed us to show that the vast majority of PfEMP1 protein was not trafficked to the surface and remains in the cytoplasm of the infected red cell. Also it was possible to monitor easily the appearance of PfEMP1 on the surface of the infected red cell during asexual development of the parasite.
The experiments presented here suggest a novel structure present at the erythrocyte membrane of infected red cells that participates in the transfer of PfEMP1 to the outside of the red cell surface. It is likely that PfEMP3 is one of a number of proteins which target this structure, and it will be of interest to identify interacting proteins which will help in understanding this important process.
Materials and methods
Plasmid construct
Plasmid pHC1-EMP3Δ3 was constructed by subcloning an XhoI fragment of PfEMP3 containing 18 repeats (Pasloske et al., 1993) from the 3′ end of PfEMP3 (Gardner et al., 1998) into the plasmid pHC1 (Crabb et al., 1997a). The insert was subcloned into pGem-T, and oligonucleotides P1 5′ CGCACTCGAGATTAGCAAGTTCAGCC 3′ (pGem-T/λgt11 linker) and P2 5′ ACCGCTCGAGTTAGGTGTATTCTTTAAATCCGTATTTG 3′ (PfEMP3) were used to amplify the XhoI 3′ repeat fragment. The XhoI sites are shown in bold. The transfection construct pHC1-EMP3Δ5U was made by inserting a 1.0 kb fragment encoding the 5′ region of the PfEMP3 gene using the oligoinucleotides PP3-5′ AGACTCGAGAGATCATTAGCCCAGGTTTTGG and PP3-3′ TTTATCTCGAGCTCTAAATTTATCAGGAAACGATG. The introduced XhoI sites are shown in bold.
Parasite culture, transfection and RT–PCR
Plasmodium falciparum parasites were cultured (Trager and Jensen, 1977) and synchronized by standard procedures. Transfections were carried out as described previously (Wu et al., 1995, 1996; Crabb and Cowman, 1996). mRNA was isolated from synchronized trophozoites and RT–PCR performed using oligonucleotide primers across DBL1 (Kyes et al., 1997) and from the SVL to ATS as described previously (Crabb et al., 1997a).
Antisera
The PfEMP3 fragment isolated for transfection was cloned into the pGEX4T-1 vector (Pharmacia, Sweden) as a GST fusion. Fusion protein was expressed, affinity purified and antibodies raised in rabbits (Smith and Johnson, 1988). Polyclonal anti-PfEMP3 antiserum was depleted of anti-GST reactivity (Crabb et al., 1997a) and purified on CNBr–Sepharose coupled to GST–EMP3. Monoclonal antibodies to PfEMP3 were generated from mice as described previously (Shulman et al., 1978). Affinity-purified rabbit anti-ATS antibodies from the PfEMP1 protein have been described previously (Crabb et al., 1997a).
Parasite extraction and western blotting
Triton X-100-insoluble/SDS-soluble proteins from the various parasite stages were extracted from the different parasite lines. A total of 5×105 parasites were loaded per well on a 6% SDS–polyacrylamide gel, followed by immunoblotting onto 0.2 µm PVDF membrane (Biorad, USA) as reported previously (Baruch et al., 1996). The filters were probed with affinity-purified rabbit anti-ATS PfEMP1 sera (1:100) or rabbit polyclonal anti-PfEMP3 sera (1:200). Detection was carried out using the Amersham ECL detection kit (Amersham, UK) following secondary detection with anti-rabbit Ig–horseradish peroxidase conjugate (Silenus, Australia).
Static and flow-based cytoadherence assays
Assays measuring the number of parasitized red blood cells bound to CD36, ICAM and chondroitin sulfate A were carried out as described previously (Crabb et al., 1997a).
Trypsin and chymotrypsin cleavage assays
Parasites were synchronized using two consecutive sorbitol treatments 4 h apart. Time zero corresponded to the second sorbitol lysis. Aliquots were removed from the culture after 4 h and divided into two samples. Preliminary work using trypsin digestion had determined that maximal cleavage was apparent after 15 min at 37°C using 100 µg/ml TPCK-treated trypsin (Worthington, USA). Control, undigested samples contained 100 µg/ml trypsin and 1 mg/ml STI (Worthington, USA), pre-incubated for 5 min at room temperature prior to addition to cultures. Trypsin digestion was performed at 37°C for 15 min. The reaction was stopped by the addition of STI to a final concentration of 1 mg/ml, mixed and incubated at room temperature for 15 min. Cultures were extracted immediately using Triton X-100 and SDS solubilization as described (Baruch et al., 1996). The equivalent of 2.5×106 parasites were loaded in each well of a 6% SDS–polyacrylamide gel, run at 200 V for 30 min and analysed by western blot using anti-ATS sera as outlined above.
Chymotrypsin sensitivity of surface PfEMP1 was determined using the same procedure as described for trypsin except that the negative control contained no protease. The reactions were stopped by quenching with the addition of Albumax.
Immunofluorescence microscopy
Synchronized parasites were cultured as described for trypsin cleavage assays except that samples were taken at 8 h intervals. Smears were air dried and fixed in 90% acetone/10% methanol. Fixed smears were incubated with affinity-purified anti-ATS (1:20), anti-PfEMP3 sera (1:30) diluted in 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), washed in PBS and incubated in sheep anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Silenus, Australia). For dual fluorescence assays, monoclonal anti-PfEMP3 hybridoma supernatant was used to dilute anti-ATS rabbit antibody (1:20). Secondary goat anti-mouse Ig–rhodamine (Chemicon, USA) and sheep anti-rabbit Ig–FITC conjugates were diluted 1:200 in 0.5% BSA and PBS. Slides were visualized using a laser confocal microscope or a Zeiss Axioskop 2 with a PCO SensiCam and video imaging software.
Surface iodination and immunoprecipitation
Mature intact parasitized erythrocytes were iodinated using the lactoperoxidase method and extracted sequentially with 1% Triton X-100 and 2% SDS as described previously (Baruch et al., 1996). Trypsinization of iodinated infected cells and immunoprecipitation using 50 µl of anti-ATS antibodies were as described (Baruch et al., 1996).
Immunoelectron microscopy
Pre-embedded trophozoites were fixed in 4% formaldehyde/PBS pH 7.4 for 1 h, washed in 0.1 M NH4Cl/0.1 M phosphate buffer for 30 min and then in PBS, and permeabilized in 0.1% Triton X-100 for 5 min. Rabbit anti-PfEMP3 serum was added (1:10) and incubated for 1 h. Fixed cells were washed and resuspended in protein A–5 nm gold (Amersham, UK) at 1:10 in 1% BSA/PBS for 1 h. Following washing, cells were fixed in 2.5% gluteraldehyde/0.1 M phosphate buffer pH 7.4 overnight at 4°C. Samples subsequently were washed in 0.1 M phosphate buffer three times, fixed with 2% osmium tetroxide in water for 1 h, washed, dehydrated in acetone and embedded in Spurr’s resin. Thin sections were stained with uranyl acetate and lead citrate. Post-embedding labelling was carried out as reported previously (Cowman et al., 1991).
Acknowledgments
Acknowledgements
We thank Sonia Caruana for expert technical assistance, David Roos for help with confocal microscopy, and Leann Tilley and Brendan Crabb for useful discussions and critical review of the manuscript. This work was supported by The National Health and Medical Research Council of Australia and the National Institutes of Health, USA (1 RO1 AI44008). J.W. is supported by a Peter Doherty fellowship from the NHMRC (Australia).
References
- Baruch D.I., Pasloske,B.L., Singh,H.B., Bi,X., Ma,X.C., Feldman,M., Taraschi,T.F. and Howard,R.J. (1995) Cloning the P.falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell, 82, 77–87. [DOI] [PubMed] [Google Scholar]
- Baruch D.I., Gormley,J.A., Ma,C., Howard,R.J. and Pasloske,B.L. (1996) Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin and intercellular adhesion molecule 1. Proc. Natl Acad. Sci. USA, 93, 3497–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch D.I., Ma,X.C., Singh,H.B., Bi,X., Pasloske,B.L. and Howard,R.J. (1997) Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood, 90, 3766–3775. [PubMed] [Google Scholar]
- Cao X., Ballew,N. and Barlowe,C. (1998) Initial docking of ER-derived vesicles required Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath,D. and Davis,R. (1986) Separation of large DNA molecules by contour clamped homogeneous electric fields. Science, 234, 1582–1585. [DOI] [PubMed] [Google Scholar]
- Cooke B.M., Berendt,A.R., Craig,A.G., MacGregor,J., Newbold,C.I. and Nash,G.B. (1994) Rolling and stationary cytoadhesion of red blood cells parasitized by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br. J. Haematol., 87, 162–170. [DOI] [PubMed] [Google Scholar]
- Corcoran L.M., Forsyth,K.P., Bianco,A.E., Brown,G.V. and Kemp,D.J. (1986) Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell, 44, 87–95. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Karcz,S., Galatis,D. and Culvenor,J.G. (1991) A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J. Cell Biol., 113, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B.S. and Cowman,A.F. (1996) Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl Acad. Sci. USA, 93, 7289–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B.S. et al. (1997a) Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell, 89, 287–296. [DOI] [PubMed] [Google Scholar]
- Crabb B.S., Triglia,T., Waterkeyn,J.G. and Cowman,A.F. (1997b) Stable transgene expression in Plasmodium falciparum. Mol. Biochem. Parasitol., 90, 131–144. [DOI] [PubMed] [Google Scholar]
- Culvenor J.G., Langford,C.J., Crewther,P.E., Saint,R.B., Coppel,R.L., Kemp,D.J., Anders,R.F. and Brown,G.V. (1987) Plasmodium falciparum: identification and localization of a knob protein antigen expressed by a cDNA clone. Exp. Parasitol., 63, 58–67. [DOI] [PubMed] [Google Scholar]
- Deitsch K.W. and Wellems,T.E. (1996) Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol. Biochem. Parasitol., 76, 1–10. [DOI] [PubMed] [Google Scholar]
- Gardner J.P., Pinches,R.A., Roberts,D.J. and Newbold,C.I. (1996) Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc. Natl Acad. Sci. USA, 93, 3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M.J. et al. (1998) Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum.Science, 282, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Kyes S., Taylor,H., Craig,A., Marsh,K. and Newbold,C. (1997) Genomic representation of var gene sequences in Plasmodium falciparum field isolates from different geographic regions. Mol. Biochem. Parasitol., 87, 235–238. [DOI] [PubMed] [Google Scholar]
- Leech J.H., Barnwell,J.W., Miller,L.H. and Howard,R.J. (1984) Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med., 159, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G.G., Warrell,M.J., White,N.J., Looareesuwan,S. and Warrell,D.A. (1985) Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol., 119, 385–401. [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Hirata,A., Ogawa,Y., Yonehara,T., Yoda,K. and Yamasaki,M. (1991) A cytoskeleton-related gene, USO1, is required for intracellular protein transport in Saccharomyces cerevisiae.J. Cell. Biol., 113, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasloske B.L., Baruch,D.I., Van Schravendijk,M.R., Handunnetti,S.M., Aikawa,M., Fujioka,H., Taraschi,T.F., Gormley,J.A. and Howard,R.J. (1993) Cloning and characterization of a Plasmodium falciparum gene encoding a novel high-molecular weight host membrane-associated protein, PfEMP3. Mol. Biochem. Parasitol., 59, 59–72. [DOI] [PubMed] [Google Scholar]
- Pologe L.G., Pavlovec,A., Shio,H. and Ravetch,J.V. (1987) Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc. Natl Acad. Sci. USA, 84, 7139–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder J.C., Cowman,A.F., Davern,K.M., Beeson,J.G., Thompson,J.K., Rogerson,S.J. and Brown,G.V. (1999) The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by PfEMP1. Proc. Natl Acad. Sci. USA, 96, 5198–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde,C.D. and Köhler,G. (1978) A better cell line for making hybridomas secreting antibodies. Nature, 276, 269–270. [DOI] [PubMed] [Google Scholar]
- Smith D.B. and Johnson,K.S. (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene, 67, 31–40. [DOI] [PubMed] [Google Scholar]
- Smith J.D., Chitnis,C.E., Craig,A.G., Roberts,D.J., Hudson-Taylor,D.E., Peterson,D.S., Pinches,R., Newbold,C.I. and Miller,L.H. (1995) Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell, 82, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Heatwole,V.M., Wertheimer,S.P., Guinet,F., Herrfeldt,J.A., Peterson,D.S., Ravetch,J.A. and Wellems,T.E. (1995) The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell, 82, 89–100. [DOI] [PubMed] [Google Scholar]
- Trager W. and Jensen,J.B. (1977) Cultivation of erythrocytic stages. Bull. WHO, 55, 363–365. [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Wang,P., Sims,P.F.G., Hyde,J.E. and Cowman,A.F. (1998) Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J., 17, 3807–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Sifri,C.D., Lei,H.-H., Su,X.-S. and Wellems,T.E. (1995) Transfection of Plasmodium falciparum within human red blood cells. Proc. Natl Acad. Sci. USA, 92, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kirkman,L.A. and Wellems,T.E. (1996) Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl Acad. Sci. USA, 93, 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]