Abstract
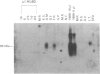
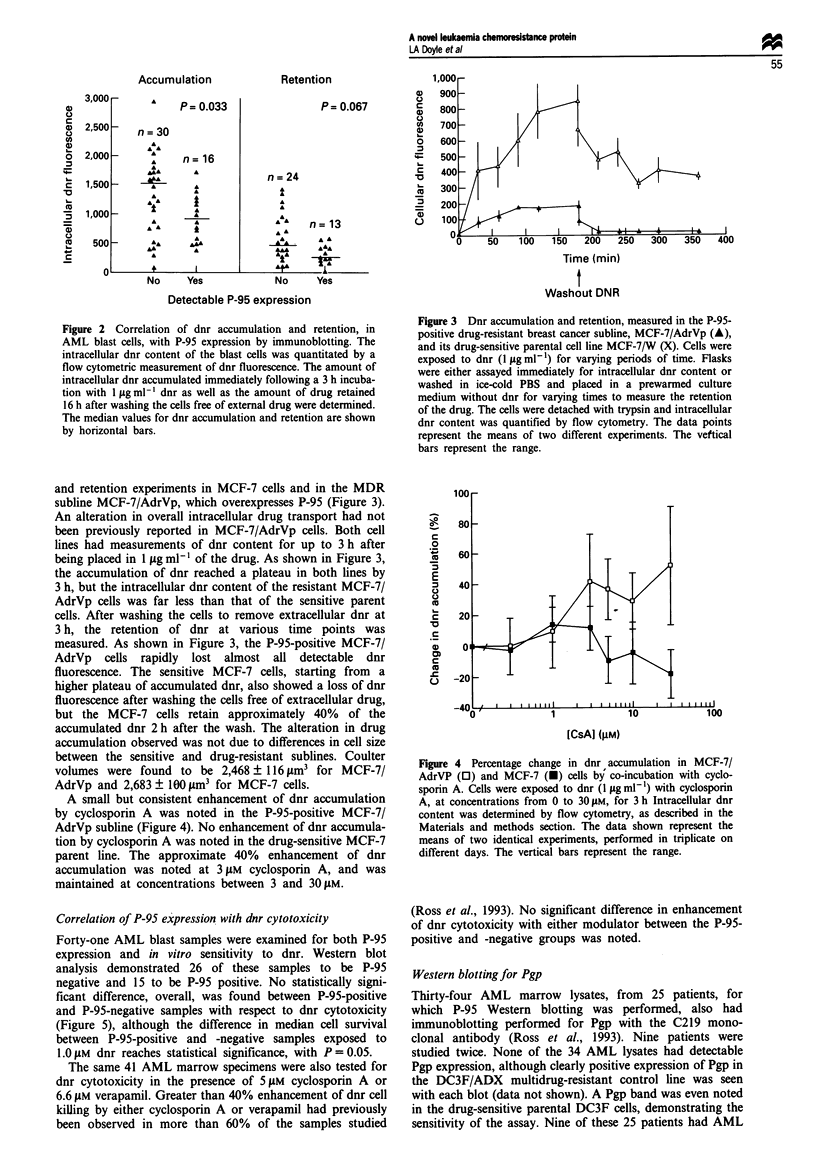
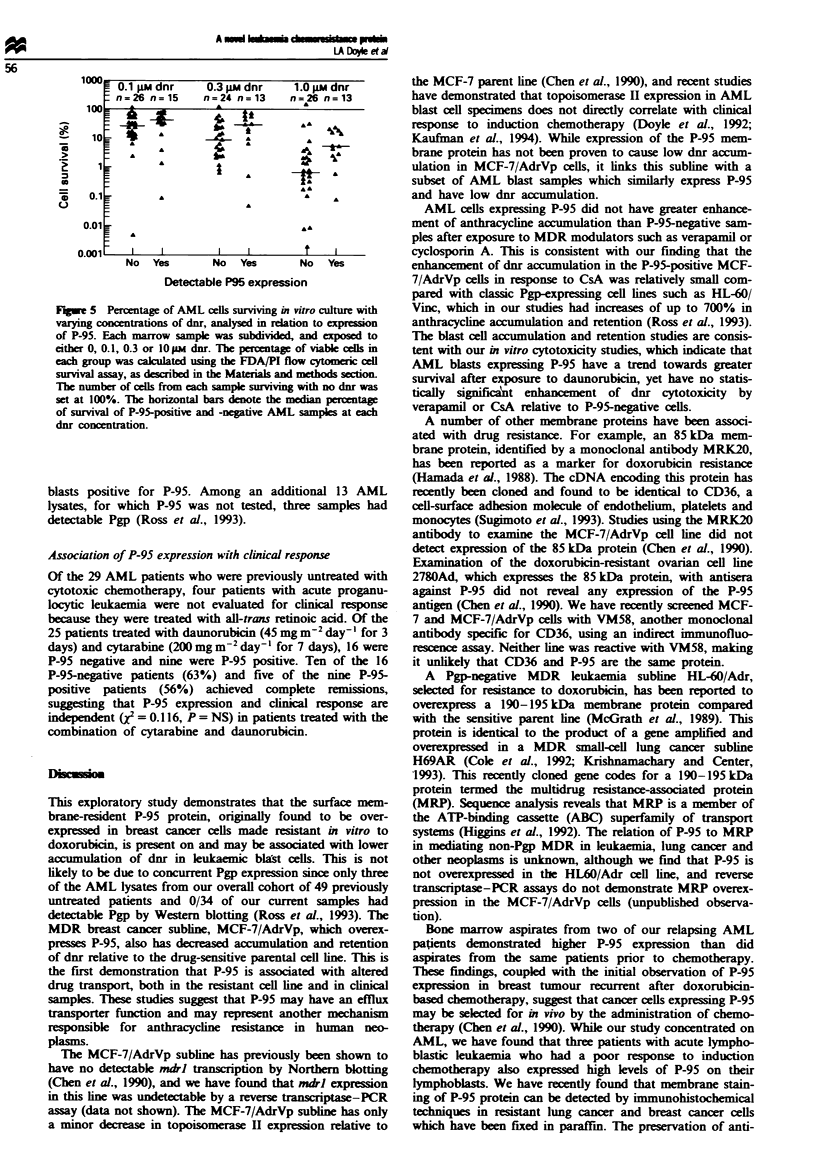
A 95 kDa membrane protein (P-95) has been previously noted to be overexpressed in a doxorubicin-resistant subline of the MCF-7 breast cancer line and in clinical samples obtained from patients with solid tumours refractory to doxorubicin. We performed Western blotting on blast cell lysates from adults with acute myeloid leukaemia, using antisera to P-95. Concomitant flow cytometric assays measured daunorubicin accumulation and retention. Blasts from 16/46 patient samples had detectable P-95 and had reduced accumulation of daunorubicin compared with the negative marrows. Experiments with the P-95 positive MCF-7 multidrug-resistant subline demonstrated decreased daunorubicin accumulation and retention relative to the sensitive parent line. AML blast cells positive for P-95 also demonstrated greater overall in vitro survival in the presence of daunorubicin relative to the P-95-negative samples. The expression of P-95 did not correlate with failure to achieve an initial complete remission with daunorubicin and cytarabine induction chemotherapy. We conclude that the P-95 protein may possess an efflux transporter function, and may represent another mechanism responsible for anthracycline resistance in acute myeloid leukaemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. N., Mickley L. A., Schwartz A. M., Acton E. M., Hwang J. L., Fojo A. T. Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J Biol Chem. 1990 Jun 15;265(17):10073–10080. [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Hamada H., Okochi E., Watanabe M., Oh-hara T., Sugimoto Y., Kawabata H., Tsuruo T. Mr 85,000 membrane protein specifically expressed in adriamycin-resistant human tumor cells. Cancer Res. 1988 Dec 15;48(24 Pt 1):7082–7087. [PubMed] [Google Scholar]
- Higgins C. F., Hyde S. C., Mimmack M. M., Gileadi U., Gill D. R., Gallagher M. P. Binding protein-dependent transport systems. J Bioenerg Biomembr. 1990 Aug;22(4):571–592. doi: 10.1007/BF00762962. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tanimoto M., Kumazawa T., Okumura M., Morishima Y., Ohno R., Saito H. Increased P-glycoprotein expression and multidrug-resistant gene (mdr1) amplification are infrequently found in fresh acute leukemia cells. Sequential analysis of 15 cases at initial presentation and relapsed stage. Cancer. 1989 Apr 15;63(8):1534–1538. doi: 10.1002/1097-0142(19890415)63:8<1534::aid-cncr2820630813>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kato S., Ideguchi H., Muta K., Nishimura J., Nawata H. Absence of correlation between cytotoxicity and drug transport by P-glycoprotein in clinical leukemic cells. Eur J Haematol. 1991 Aug;47(2):146–151. doi: 10.1111/j.1600-0609.1991.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Karp J. E., Jones R. J., Miller C. B., Schneider E., Zwelling L. A., Cowan K., Wendel K., Burke P. J. Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood. 1994 Jan 15;83(2):517–530. [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Marie J. P., Zittoun R., Sikic B. I. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood. 1991 Aug 1;78(3):586–592. [PubMed] [Google Scholar]
- Marquardt D., McCrone S., Center M. S. Mechanisms of multidrug resistance in HL60 cells: detection of resistance-associated proteins with antibodies against synthetic peptides that correspond to the deduced sequence of P-glycoprotein. Cancer Res. 1990 Mar 1;50(5):1426–1430. [PubMed] [Google Scholar]
- McGrath T., Latoud C., Arnold S. T., Safa A. R., Felsted R. L., Center M. S. Mechanisms of multidrug resistance in HL60 cells. Analysis of resistance associated membrane proteins and levels of mdr gene expression. Biochem Pharmacol. 1989 Oct 15;38(20):3611–3619. doi: 10.1016/0006-2952(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. D., Wooten P. J., Sridhara R., Ordóez J. V., Lee E. J., Schiffer C. A. Enhancement of daunorubicin accumulation, retention, and cytotoxicity by verapamil or cyclosporin A in blast cells from patients with previously untreated acute myeloid leukemia. Blood. 1993 Aug 15;82(4):1288–1299. [PubMed] [Google Scholar]
- Rothenberg M., Ling V. Multidrug resistance: molecular biology and clinical relevance. J Natl Cancer Inst. 1989 Jun 21;81(12):907–910. doi: 10.1093/jnci/81.12.907. [DOI] [PubMed] [Google Scholar]
- Sato H., Preisler H., Day R., Raza A., Larson R., Browman G., Goldberg J., Vogler R., Grunwald H., Gottlieb A. MDR1 transcript levels as an indication of resistant disease in acute myelogenous leukaemia. Br J Haematol. 1990 Jul;75(3):340–345. doi: 10.1111/j.1365-2141.1990.tb04346.x. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Hamada H., Tsukahara S., Noguchi K., Yamaguchi K., Sato M., Tsuruo T. Molecular cloning and characterization of the complementary DNA for the M(r) 85,000 protein overexpressed in adriamycin-resistant human tumor cells. Cancer Res. 1993 Jun 1;53(11):2538–2543. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]