Abstract
Cerebellar long-term depression (LTD) is thought to play an important role in certain types of motor learning. However, the molecular mechanisms underlying this event have not been clarified. Here, using cultured Purkinje cells, we show that stimulations inducing cerebellar LTD cause phosphorylation of Ser880 in the intracellular C-terminal domain of the AMPA receptor subunit GluR2. This phosphorylation is accompanied by both a reduction in the affinity of GluR2 to glutamate receptor interacting protein (GRIP), a molecule known to be critical for AMPA receptor clustering, and a significant disruption of postsynaptic GluR2 clusters. Moreover, GluR2 protein released from GRIP is shown to be internalized. These results suggest that the dissociation of postsynaptic GluR2 clusters and subsequent internalization of the receptor protein, initiated by the phosphorylation of Ser880, are the mechanisms underlying the induction of cerebellar LTD.
Keywords: AMPA receptor/cluster/GluR2/LTD/Purkinje cell
Introduction
Ionotropic glutamate receptors mediate fast excitatory synaptic transmission in the vertebrate central nervous system and have been implicated in neuronal development and synaptic plasticity, including learning and memory (Ito, 1989; McDonald and Johnston, 1990; Bliss and Collingridge, 1993). Based on pharmacological and molecular biological studies, glutamate receptors are classified into four distinct subfamilies: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (subunits GluR1–4), kainate receptors (GluR5–7 and KA1–2), NMDA receptors (NR1 and NR2A–D) and δ glutamate receptors (δ1–2).
AMPA receptors are tetramers composed of a combination of the subunits GluR1–4, with their subunit stoichiometry depending on the expression level of the respective genes (Rosenmund et al., 1998) and, hence, differing between cell types. Cerebellar Purkinje neurons receive excitatory input from the granule cells; these synapses contain AMPA receptors with a high proportion of GluR2(/3) but few GluR1 subunits (Kano et al., 1996; Zhao et al., 1998). When Purkinje cells receive conjunctive inputs from parallel fibers, axons of granule cells and climbing fibers, i.e. axons of other afferent neurons in the inferior olive (Ito, 1989), neurotransmission at granule cell–Purkinje cell synapses exhibits a long-lasting depression (long-term depression, LTD), which has been proposed to underlie several forms of motor learning (Thompson, 1986; Ito, 1989, 1990). Accumulating evidence has revealed that activation of protein kinase C (PKC) is essential for the induction of LTD (Crepel and Krupa, 1988; Ito, 1989; Ito and Karachot, 1989, 1990, 1992; Linden and Connor, 1991). Consequently, PKC-mediated phosphorylation of GluR2 (or GluR3), highly concentrated at the postsynaptic sites of Purkinje cell dendrites, has been hypothesized to play an important role in the induction of LTD by modulating AMPA receptor channel function.
Recent studies have shown that the C-terminal region of glutamate receptor subunits constitutes the major intracellular domain of these transmembrane proteins (Hollmann et al., 1994; Bennet and Dingledine, 1995; Hirai et al., 1996). Based on this finding, we previously examined phosphorylation of the C-terminal domain of GluR2 and identified Ser880 as the PKC phosphorylation site (Matsuda et al., 1999). Moreover, phosphorylation of Ser880 was found to prevent the C-terminus of GluR2 from binding to the glutamate receptor interacting protein (GRIP) both in vitro and in transfected human embryonic kidney (HEK) 293 cells (Matsuda et al., 1999). Since the interaction between GluR2 and GRIP is crucial for precise apposition of GluR2 to the postsynaptic membrane (Dong et al., 1997), phosphorylation of Ser880 in GluR2 affects GluR2 distribution and/or clustering in the postsynaptic membrane. We therefore proposed that cerebellar LTD might be caused by the disruption of postsynaptic AMPA receptor clusters that results from a loss of anchoring via GRIP. In the present study, we used cultured cerebellar Purkinje neurons to show that stimulations inducing LTD cause modulation of the postsynaptic GluR2 cluster density via PKC phosphorylation of Ser880. Our data provide strong evidence that cerebellar LTD results from a decrease in postsynaptic AMPA receptor density.
Results
Induction of LTD at granule cell–Purkinje cell synapses following PKC activation
It has been shown that PKC activation by phorbol ester can induce LTD of glutamate responses of cultured Purkinje neurons (Linden and Connor, 1991). Since in these experiments glutamate was applied iontophoretically, we examined whether PKC activation could cause depression of synaptic transmission between granule cell axons and Purkinje cell dendrites. Excitatory postsynaptic current (EPSC) in a Purkinje cell was evoked by electrical stimulation of a single granule cell. As shown in Figure 1, bath-application of 12-tetradecanoyl phorbol 13-acetate (TPA) led to a significant reduction of the EPSC amplitude, to ∼40% of its original level after 20 min of treatment. This indicates that PKC activation induces LTD of synaptic transmission between cultured granule cells and Purkinje cells.
Fig. 1. Induction of cerebellar LTD by PKC activation. Whole-cell voltage-clamp recording was performed from a Purkinje cell at 25 days in vitro. EPSC was evoked by extracellular stimulation of a single granule cell. (A) Amplitudes of the evoked EPSC, plotted as a function of time relative to the beginning of TPA application (200 nM TPA at 0 min). Each data point represents the amplitude of one evoked EPSC, as a percentage of the control amplitude. The average of the EPSC amplitude before TPA application was set at 100%. Insets show the representative current traces taken at times (a), (b) and (c), respectively. (B) Each point represents the normalized EPSC amplitude from five Purkinje cells (recording condition as above). The EPSC amplitudes were averaged over a 2 min time window. Bars indicate SD.
Regulation of GluR2 localization in HEK 293 cells
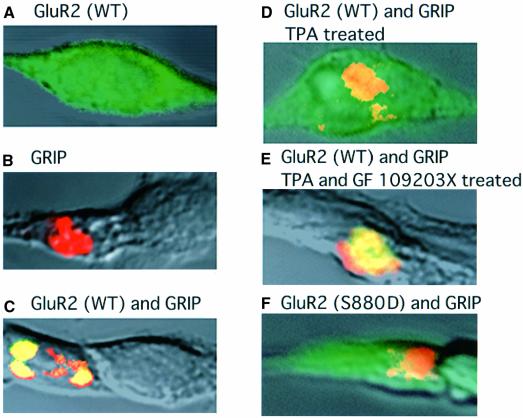
Using heterologous expression, we have shown previously that PKC phosphorylates Ser880 in the C-terminal domain of GluR2, and that this phosphorylation reduces the affinity of the receptor to the GRIP fragment containing PDZ 4–5 (amino acids 419–673) (GRIP419–673) (Matsuda et al., 1999), a region essential and sufficient for binding to GluR2 (Dong et al., 1997). These results suggest that phosphorylation of Ser880 in GluR2 may regulate the postsynaptic localization of AMPA receptors by inhibiting GRIP binding. Here, we examined the effect of Ser880 phosphorylation on the cellular localization of GluR2 by expressing GluR2 and GRIP419–673 in transfected HEK 293 cells. When either GluR2 subunit or a His-tagged GRIP419–673 was expressed singly in HEK 293 cells, confocal microscopy revealed that the immunoreactive receptor protein was diffusely distributed in the cytosol (Figure 2A), whereas that of GRIP419–673 aggregated in cytoplasmic macroclusters (Figure 2B). Co-expression of GluR2 with GRIP419–673 caused clustering of the GluR2 protein at the sites of aggregation of GRIP419–673 (Figure 2C). This co-localization of GluR2 with GRIP419–673 was prevented upon activation of endogenous PKC with 200 nM TPA for 20 min (Figure 2D). The effect of TPA was completely blocked by co-application of a selective PKC inhibitor, GF109203X (200 nM) (Figure 2E). Moreover, substitution of the Ser880 residue in GluR2 by aspartate, which mimicks phosphorylation, prevented the co-localization with GRIP (Figure 2F). These results indicate that phosphorylation of Ser880 regulates the subcellular localization of GluR2 by controlling its binding to GRIP.
Fig. 2. Effect of PKC activation on the co-localization of GluR2 and GRIP in transfected HEK 293 cells. (A and B) HEK cells expressing either GluR2 (FITC labeled) (A) or GRIP419–673 (rhodamine labeled) (B). (C and D) HEK cells simultaneously expressing GluR2 and the GRIP fragment prior to (C) and after (D) TPA stimulation (200 nM, 20 min). (E) A cell expressing GluR2 and the GRIP419–673 20 min after treatment with TPA and GF109203X (200 nM), a selective PKC inhibitor. (F) A cell expressing mutant GluR2 (S880D) and GRIP419–673.
Regulation of postsynaptic GluR2 clusters on Purkinje cell dendrites
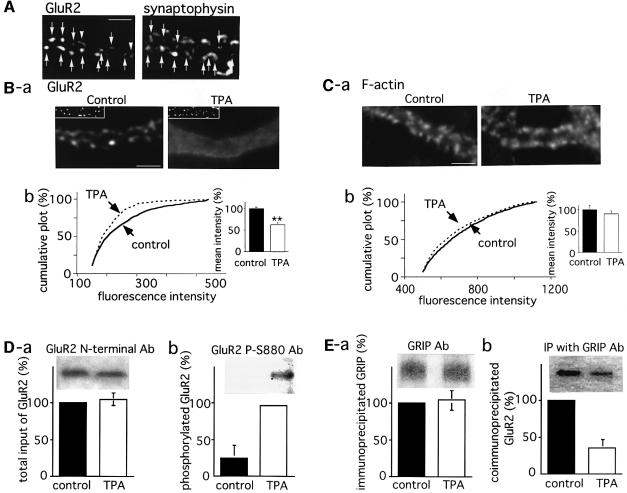
The GluR2 protein and GRIP are known to co-localize in the dendritic spines of Purkinje cells (Wyszynski et al., 1998; Burette et al., 1999). On the other hand, immunocytochemical examination with anti-GluR2 N-terminal and anti-synaptophysin antibodies (Figure 3A) revealed that most of the GluR2 immunoreactive clusters were co-localized with those of synaptophysin (86.7 ± 3.1%, 300 clusters in three cells examined). This indicates that the majority of the GluR2 clusters are localized at excitatory postsynaptic sites. To examine whether Ser880 in GluR2 is phosphorylated upon PKC activation in native Purkinje neurons, and whether this phosphorylation disrupts the clusters of GluR2 on the spine membrane, we treated cultured Purkinje cells with TPA (200 nM, 20 min) followed by staining with anti-GluR2 N-terminal antibodies. In the control cultures, GluR2 immunoreactive clusters were observed along the dendrites (Figure 3Ba, left panel). On the other hand, the fluorescence intensities of the GluR2 clusters in TPA-stimulated cells were markedly reduced without a corresponding decrease in synaptophysin immunoreactivity (Figure 3Ba, right panel). In order to confirm the effect of TPA on GluR2 clusters statistically, the images were filtered and GluR2 clusters defined by thresholding, followed by measurement of the maximum intensity of each cluster using IPLab imaging software (Scanalytics, Inc.). Figure 3Bb shows the cumulative plot of the maximum intensities of the GluR2 clusters. The intensities of the GluR2 clusters were significantly reduced by TPA stimulation (Kolmogorov–Smirnov test, p <0.0001). The inset bar graph in Figure 3Bb shows the mean of the maximum cluster intensities, determined as described in Materials and methods, again indicating a significant decrease following TPA treatment. A similar analysis of fluorescence images obtained by staining with rhodamine–phalloidin revealed that F-actin clusters in the dendritic spines were not significantly affected upon TPA application (Figure 3Ca and b). These results indicate that PKC stimulation with TPA causes considerable disruption of GluR2 clusters without significant effects on the spine morphology.
Fig. 3. Disruption of GluR2 clusters in response to PKC activation. A cerebellar neuronal culture at 14 DIV was used for the following analysis. (A) Co-localization of GluR2 and a presynaptic marker synaptophysin. The cerebellar neuronal culture was double immunostained with anti-GluR2 N-terminal (left) and anti-synaptophysin (right) antibodies. Arrows indicate the spots representing immunoreactivity for both antibodies. Arrowheads indicate the GluR2- but not synaptophysin-immunoreactive spots. (B and C) Disruption of the postsynaptic GluR2 clusters following TPA application. Purkinje cells were treated with 200 nM TPA for 20 min, followed by staining with anti-GluR2 N-terminal antibodies (B) or rhodamine–phalloidin (C). The GluR2 and F-actin clusters were defined after filtering the images, followed by quantification of the maximum intensity of each cluster (see Materials and methods). Images were obtained from the dendritic regions of control (left) and TPA-treated (right) cells (scale bars 5 µm). Insets in (Ba) show fluorescence images for synaptophysin. Graphs (Bb) are cumulative plots of the maximum intensities for GluR2 (B) and F-actin (C) clusters. Solid and dashed lines represent the results from control and TPA-treated cells, respectively. The Kolmogorov–Smirnov test revealed a significant decrease in the intensity of GluR2 clusters following TPA stimulation (p <0.0001). Bar graphs in the inset show the mean of maximum cluster intensities obtained from five different cells. The intensity of the control culture was taken as 100%. Asterisks indicate that the mean intensity of the GluR2 clusters in TPA-treated cells was significantly lower than that in control cells (**p <0.01). (D) Phosphorylation of Ser880 in GluR2 following application of TPA. The membrane fraction of cultured Purkinje cells treated or not treated with TPA (200 nM, 20 min) was subjected to SDS–PAGE and immunoblotting with anti-GluR2 N-terminal antibodies (a) or antibodies specific for the phosphoSer880 residue in GluR2 (anti-P-Ser880 antibodies) (b). (E) Parallel decrease in the affinity of GluR2 for GRIP. Immunoprecipitation was performed using polyclonal anti-GRIP antibodies. Immunoprecipitated GRIP (a) and co-immunoprecipitated GluR2 (b) were detected by immunoblotting with anti-GRIP and anti-GluR2 N-terminal antibodies, respectively. Bar graphs in (D) and (E) show the mean of the band intensities (n = 3). Error bars indicate SD.
Immunoblot analysis using polyclonal antibodies specific for the phosphoserine at position 880 in GluR2 (anti-P-Ser880 antibodies) revealed that TPA treatment significantly increased the level of GluR2 phosphoryl ation (Figure 3Db). Immunoblotting with anti-GluR2 N-terminal antibodies revealed that similar amounts of GluR2 protein were loaded (Figure 3Da). The lower portion of Figure 3Db shows a quantitative analysis (n = 3) of phosphoserine antibody binding normalized to the amount of GluR2 protein, indicating an ∼4-fold increase in phosphorylation levels upon TPA treatment. Parallel immunoprecipitation experiments with anti-GRIP antibodies revealed a significant decrease in the amount of co-immunoprecipitated GluR2 to ∼40% of that observed in the case of non-treated control cultures (Figure 3E). This is clearly indicative of reduced GluR2 binding to GRIP. Collectively, these results suggest that the disruption of GluR2 clusters by TPA, via PKC phosphorylation of Ser880, results from a loss of anchoring via GRIP.
The potentiative effect of AMPA on TPA-mediated GluR2 declustering and GluR2 Ser880 phosphorylation
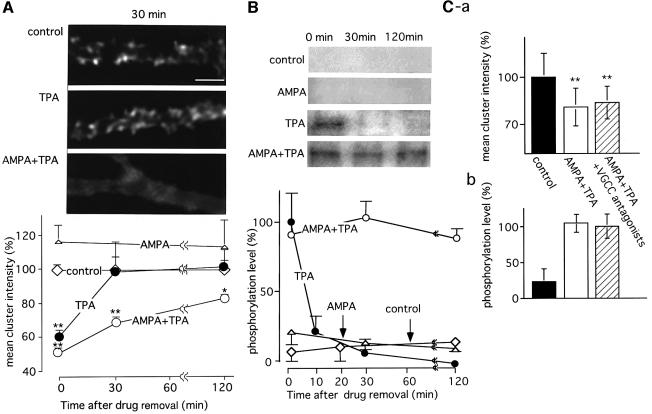
Our data show that PKC activation by TPA for 20 min causes disruption of postsynaptic GluR2 clusters. To determine the duration for which GluR2 phosphorylation and declustering is maintained after removal of the drug, and whether dephosphorylation and reclustering of GluR2 occur, we examined both the distribution of GluR2 immunoreactivity on dendrites and the phosphorylation of Ser880 after washing out TPA. Changes in receptor clustering were evaluated first by calculating the means of the maximal cluster intensities. As shown in Figure 4A, clustering of GluR2 was completely restored 30 min after removal of TPA. A previous study demonstrated that treatment of cultured Purkinje cells with phorbol ester alone is less effective at inducing LTD than conjunctive stimulation of TPA with AMPA (Linden and Connor, 1991). We therefore co-applied AMPA (100 µM, 1 min) at the start of TPA (200 nM, 20 min) treatment. This caused efficient disruption of the GluR2 clusters, with a decrease in the mean cluster intensity to a level similar to that in cells treated with TPA alone. However, in contrast to the cells treated with TPA alone, the decrease in mean GluR2 cluster intensity, i.e. the disruption of AMPA receptor clusters, persisted for >2 h even though treatment of the cells with AMPA alone had no effect (Figure 4A). Subsequently, the levels of Ser880 phosphorylation following stimulation with TPA, AMPA and TPA+AMPA were examined. The results obtained showed good correlation with the observed changes in GluR2 cluster intensity. The phosphorylation level, which increased following TPA stimulation, returned to its basal level within 30 min; treatment with AMPA alone had no effect. In contrast, after combined application of AMPA and TPA, phosphorylation was maintained for >2 h (Figure 4B). These results are consistent with those of Linden and Connor (1991), who demonstrated that application of AMPA, in addition to phorbol ester stimulation, results in efficient induction of LTD in cultured Purkinje cells. Since AMPA stimulation is considered to depolarize Purkinje cells and consequently to increase intracellular Ca2+ concentration via the opening of voltage-gated Ca2+ channels (VGCCs), the elevated intracellular Ca2+ concentration through this pathway might play an important role in preventing the reclustering as well as the dephosphorylation of GluR2. Then, we examined whether co-application of VGCC antagonists with TPA+AMPA canceled the effect of AMPA on those two parameters. However, application of both verapamil (10 µM) and ω-agatoxin TK (50 nM), which has been shown to block >90% of the Ca2+ currents via VGCCs in rat Purkinje cells (Regan, 1991; Mintz and Bean, 1993), did not significantly affect the results caused by TPA+AMPA (Figure 4C). These results suggest that VGCCs do not contribute to the AMPA-induced potentiation or the effect of VGCCs is compensated by intracellular Ca2+ increase via a different pathway.
Fig. 4. Long-lasting disruption of GluR2 clusters in association with persistent phosphorylation of the Ser880 residue in GluR2 following conjunctive stimulation with TPA and AMPA. (A) Time course of changes in the density of GluR2 clusters. Cerebellar neuronal cultures were stimulated with TPA (200 nM, 20 min), AMPA (100 µM, 1 min) or TPA+AMPA, and incubated for 0, 30 and 120 min after the removal of the drugs. Then, the cells were fixed and immunostained for GluR2. Upper panels indicate the disruption of GluR2 immunoreactivity on Purkinje cell dendrites at 30 min after removal of the drugs (scale bar 5 µm). For quantitative analysis of the effect of TPA on GluR2 cluster density, the mean of the maximum cluster intensities was calculated from five different cells. The results are plotted on the graph, with the mean intensity in the control culture being 100%. (B) Time course of changes in Ser880 phosphorylation levels following various stimulations. Cerebellar neuronal cultures were treated as described above. After a set period of incubation, the cells were solubilized, and the membrane fractions were subjected to SDS–PAGE, followed by immunoblotting with anti-P-Ser880 antibodies (insets). The means of the band intensities from three independent experiments are plotted on the graph. The band intensities obtained from TPA-treated cultures without further incubation were defined as 100%. Error bars indicate SD. (C) No significant effect of VGCC blockers on TPA+AMPA induced disruption of the GluR2 clusters and phosphorylation of Ser880 in GluR2. TPA+AMPA stimulation was applied in the presence or absence of VGCC blockers, verapamil (10 µM) and ω-agatoxin TK (50 nM), and incubation was carried out for 30 min after the removal of drugs. Then, mean cluster intensity (a) and the phosphorylation level (b) of GluR2 were quantified. Asterisks indicate the significant difference as compared with control samples (*p <0.05, **p <0.01).
Disruption of GluR2 clusters and GluR2 Ser880 phosphorylation by a different protocol inducing cerebellar LTD
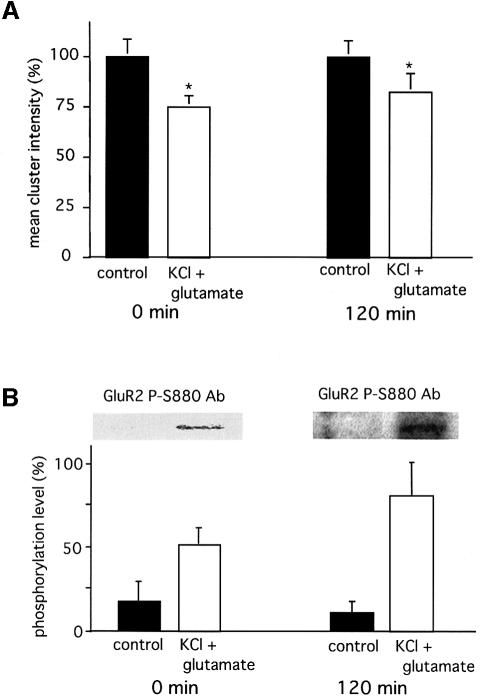
Another group has demonstrated that activation of glutamate receptors together with depolarization induced by KCl also efficiently induces LTD in cultured Purkinje cells (Kawasaki et al., 1999). To elucidate whether molecular events similar to those observed after stimulation with AMPA and TPA underlie LTD induction by glutamate and KCl, cultured Purkinje cells were treated with 10 µM glutamate and 50 mM KCl for 4 min. This treatment also resulted in a long-lasting disruption of GluR2 clusters (Figure 5A) in association with persistent phosphorylation of Ser880 (Figure 5B) for up to 2 h after the removal of the drugs. We therefore conclude that phosphorylation of Ser880 by PKC provides a general mechanism for LTD induction.
Fig. 5. Disruption of GluR2 clusters concurrently with phosphorylation of the Ser880 residue in GluR2 following KCl + glutamate stimulation. A cerebellar neuronal culture was stimulated with 50 mM KCl together with 10 µM glutamate for 4 min. Then, the drugs were removed and used for subsequent analysis immediately or after 2 h of incubation. (A) Quantitative analysis of GluR2 cluster intensities. The graph shows the means of the maximal cluster intensities obtained from five different cells fixed immediately or 2 h after KCl + glutamate stimulation (white columns). Black columns show the results from control samples. The mean cluster intensities obtained from control cultures were defined as 100%. Asterisks indicate that the mean intensity of the GluR2 clusters in the stimulated cells was statistically significantly weaker than that in control cells (*p <0.05). (B) Phosphorylation of the Ser880 residue in GluR2 following KCl + glutamate stimulation. Cerebellar neuronal cultures treated (white columns) or not treated (black columns) with KCl + glutamate, and incubated for 0 or 120 min after removal of the drugs. The membrane fractions were subjected to SDS–PAGE and subjected to immunoblotting with anti-P-Ser880 antibodies (insets). The lower graph shows the mean of the band intensities after normalization with loaded GluR2 protein (n = 3). Similar experiments with TPA stimulation (200 nM, 20 min, no further incubation) were performed simultaneously and the band intensities were taken as 100%. Error bars indicate SD.
Internalization of GluR2 protein in Purkinje cells following PKC activation
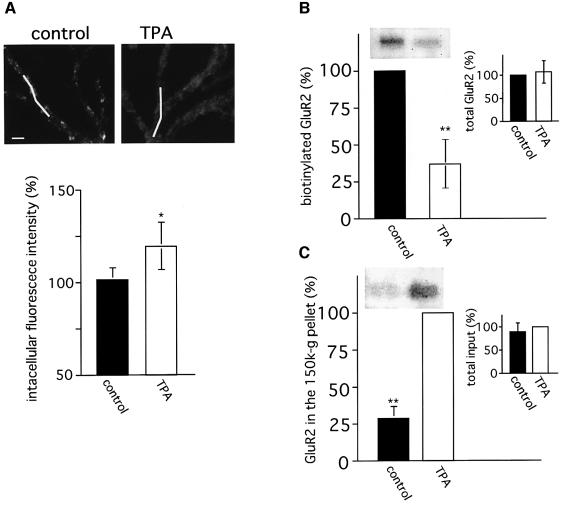
Where do AMPA receptors that have lost anchoring go? To the extrasynaptic area along the plasma membrane or into the cytoplasm by internalization? To answer these questions, we evaluated the change in intracellular GluR2 immunoreactivity by confocal microscopy. As shown in Figure 6A, intracellular GluR2 immunoreactivities are significantly increased upon TPA stimulation, suggesting the internalization of GluR2 protein following PKC activation. This notion is supported by two different biochemical analyses. One is cell surface biotinylation, which allows the determination of the relative amounts of extracellularly expressed GluR2 protein by the following procedure. Cell surface proteins are biotinylated, and total GluR2 is immunoprecipitated with anti-GluR2 C-terminal antibodies. Then, the relative amounts of biotinylated, namely extracellularly expressed, GluR2 are quantified by immunoblotting with streptavidin. Figure 6B shows the GluR2 biotinylation level when the value in non-treated cells is assumed to be 100%. A significant reduction of extracellularly expressed GluR2 by ∼40% is observed upon stimulation with TPA. Another assay is subcellular fractionation (Delft et al., 1997). After TPA stimulation of the cultured cells, a fraction (150 000 g fraction) containing endocytosed clathrin-coated vesicles is isolated, and the relative amount of GluR2 protein in this fraction is determined by immunoblotting for GluR2. As shown in Figure 6C, the amount of GluR2 is significantly increased following TPA stimulation. Although GluR2 is expressed not only in Purkinje cells, but also in granule cells, a previous immunohistochemical study (Burette et al., 1999) and our immunocytochemical studies (data not shown) show that a majority of the GluR2 proteins in the cerebellum are expressed in Purkinje cells. Then, the drastic decrease in the quantity of cell-surface-expressed GluR2, and the corresponding increase in the amount of GluR2 in a 150 000 g fraction, are considered to result mainly from the internalization of GluR2 protein in Purkinje cells. Collectively, these results strongly suggest that a significant amount of GluR2 protein is internalized in Purkinje cells via clathrin-mediated endocytosis, following the stimulation inducing cerebellar LTD.
Fig. 6. Internalization of GluR2 protein following PKC activation. (A) Increase in intensity of immunoreactive intradendritic GluR2. Upper panels show respective confocal optical sections of the dendritic regions in control and TPA-treated Purkinje cells immunostained after permeabilization treatment with anti-GluR2 N-terminal antibodies (scale bar 5 µm). White bars indicate the region whose immunoreactive intensities were quantified. The graph indicates results of the quantitative analysis of intracellular immunoreactive intensities (five cells, total ∼120 µm dendritic length). The mean intracellular intensities obtained from control cultures were defined as 100%. (B) Decrease in the amount of extracellularly expressed GluR2 following TPA stimulation. Cell surface proteins were biotinylated followed by immunoprecipitation for GluR2. Then, the biotinylated GluR2 protein was detected by immunoblotting with HRP-conjugated streptavidin. Inset graph indicates the total amount of immunoprecipitated GluR2 protein. (C) Increase in the amount of GluR2 protein contained in the 150 000 g fraction following TPA stimulation. A 150 000 g fraction containing clathrin-coated vesicles was obtained as described in Materials and methods. The amount of GluR2 in this fraction was quantified by immunoblot analysis using anti-GluR2 N-terminal antibodies. The inset graph indicates the results of the quantitative analysis of total GluR2 protein contained in respective cultures. The band intensities obtained from control (B) or TPA-treated (C) cultures were taken as 100%. Asterisks in (A)–(C) indicate that the difference in GluR2 immunoreaction between control and TPA-treated samples was statistically significant (*p <0.05, **p <0.01).
Discussion
The major finding of this work is that stimulations inducing LTD at granule cell–Purkinje cell synapses cause phosphorylation of Ser880 in the C-terminal region of GluR2 and thereby reduce the binding affinity of GluR2 to GRIP, a protein known to be important for AMPA receptor clustering. Moreover, phosphorylation of Ser880 in GluR2 is shown to correlate well with disruption of postsynaptic GluR2 clusters in cultured Purkinje cells.
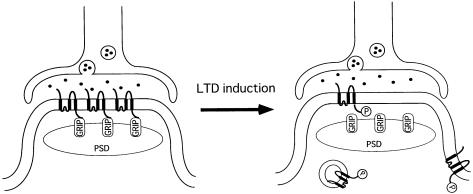
Our data can be summarized in a model that explains how the number of postsynaptic AMPA receptors containing the GluR2 subunit can be modulated (Figure 7). The C-terminus of GluR2 is anchored to GRIP, a protein component of the postsynaptic density (Figure 7, left). Upon LTD induction, Ser880 of GluR2 is phosphorylated by PKC, leading to dissociation of GluR2 from GRIP and a decreased density of GluR2-containing AMPA receptors at the postsynaptic membrane (Figure 7, right) by internalization of the receptor protein. Different results also support the internalization hypothesis. First, in the experiments on cerebellar LTD by Linden and colleagues (Linden and Connor, 1991; Linden, 1995), Purkinje cell responses were rapidly induced by iontophoretic application of glutamate; the decrease in the agonist-induced current is therefore more likely to be caused by internalization of the receptors rather than lateral diffusion. Secondly, it has been demonstrated that synaptic AMPA receptors are recycled in hippocampal neurons with a time course of minutes using exocytotic and endocytotic pathways, and that blockade of exocytosis by different pharmacological treatments causes depression of the AMPA receptor-mediated EPSCs. After stabilization of the pharmacological depression of the EPSCs, LTD can no longer be elicited, suggesting the occlusion of these two events (Lüscher et al., 1999). On the other hand, tetanic synaptic stimulation inducing LTP has been shown to facilitate delivery of the GluR1 receptor protein to the postsynaptic membrane in hippocampal CA1 neurons (Shi et al., 1999). These results, together with our present data, suggest that clathrin-mediated AMPA receptor endocytosis underlies the induction of LTD at parallel fiber–Purkinje neuron synapses.
Fig. 7. A model depicting LTD of AMPA receptor response at parallel fiber–Purkinje cell synapses. The left part of the figure shows the basal state of the synapse. The intracellular C-terminal tail of GluR2 is bound to GRIP, and thus immobilized at the postsynaptic membrane. The right part of the figure shows the synapse after induction of LTD. Ser880 in the C-terminal domain of GluR2 is phosphorylated, which causes dissociation of GluR2 from GRIP, resulting in a decrease in the density of functional AMPA receptors in the postsynaptic membrane. PSD, postsynaptic density; GRIP, glutamate receptor interacting protein.
The model depicted in Figure 7 is thought to be not entirely applicable to LTD in other brain regions since the subunit composition of AMPA receptors and the corresponding anchoring proteins expressed differ from region to region: the association between a receptor subunit and an anchoring protein may not be affected by PKC phosphorylation. In addition, a previous immunohistochemical study revealed the dissociation of GRIP and GluR2 distribution in the pyramidal neurons of the cerebral cortex, although perfect co-localization of GRIP and GluR2 was observed in the dendrites of Purkinje cells (Burette et al., 1999). These suggest that GRIP contributes little to the postsynaptic GluR2 clustering in the cerebral neurons and, hence, our model does not seem to apply to this neuron type. However, we assume that the mechanism involved in the regulation of synaptic efficacy by the dynamic change in postsynaptic receptor density is not limited to parallel fiber–Purkinje cell synapses, and other synapses in different brain regions utilize similar mechanisms involving clathrin-mediated endocytosis, as recently demonstrated in the hippocampus (Lüscher et al., 1999; Shi et al., 1999).
Our results corroborate the importance of AMPA receptor stimulation for the effective induction of cerebellar LTD and are fully consistent with the results of a previous study by Linden and Connor (1991), in which LTD was effectively induced upon co-stimulation with both AMPA and phorbol ester, although in cultures treated with phorbol ester alone, ∼70% of the cells failed to show depression of the glutamate current 20 min after removal of the drug. The molecular mechanisms that contribute to LTD downstream of AMPA receptor activation are unknown. Blockade of VGCCs was shown to cause no significant effect on AMPA-induced potentiation (Figure 4C). However, since AMPA stimulation causes influx of Na+, thus leading to an increase in the intracellular calcium concentration via inhibition of the Na+–Ca2+ exchanger, one possible role of AMPA in LTD induction may be to suppress the activity of phosphatases to allow the phosphorylation of Ser880 in GluR2 to be maintained. This idea is supported by previous reports showing that climbing fiber input, which causes strong depolarization of the Purkinje cells, is, in addition to parallel fiber stimulation, essential for the electrical induction of LTD (Ito, 1989). Also, application of the protein phosphatase inhibitors calyculin A or microcystein-LR has been shown to induce LTD in cerebellar slices in response to parallel fiber stimulation alone and, thus, to substitute for the climbing fiber signals (Ajima and Ito, 1995). Alternatively, synergistic enhancement of PKC activity by unsaturated fatty acids such as arachidonate and oleate might be involved in the mechanisms that underlie robust LTD induction by AMPA and phorbol ester, since phospholipase A2 can be activated by the Ca2+ influx following AMPA stimulation; the active enzyme then cleaves membrane phospholipids to produce unsaturated fatty acids (Dennis et al., 1991). Actually, the contribution of this phospholipase A2-mediated pathway to the induction of LTD in cultured Purkinje cells has been demonstrated previously (Linden, 1995).
The present results seem to explain clearly the molecular events that occur during the early phase (∼2 h) of cerebellar LTD. However, other mechanisms such as protein synthesis or activation of CaMKIV have been shown to be essential for the maintenance of late-phase LTD (Linden, 1996; Ahn et al., 1999). Therefore, the possibility that activated PKC triggers a different pathway for the transition from early- to late-phase LTD cannot be excluded. This is a topic for further study.
During the preparation of this manuscript, a paper was published by Wang and Linden (2000), who showed using electrophysiology that the expression of cerebellar LTD requires a clathrin-mediated endocytotic process. Their finding strongly supports our conclusion that AMPA receptors that lose their anchoring to GRIP are internalized via the clathrin-mediated endocytotic pathway.
Here, we have demonstrated a significant correlation between GluR2 Ser880 phosphorylation and the disruption of GluR2 clusters in Purkinje dendrites following stimulations inducing LTD. A significant amount of the receptor protein was considered to be internalized. Our results provide a basis for elucidating in greater detail the molecular mechanism underlying cerebellar LTD.
Materials and methods
Electrophysiology
The experiments were carried out with 3- to 4-week cultures. The recording chamber was clamped on the stage of a Nikon TE300 inverted microscope and observed with phase-contrast optics. The preparations were continuously superfused with an extracellular solution. The extracellular solution contained (in mM) NaCl 140, KCl 3, CaCl2 3, MgCl2 1.5, glucose 10, HEPES 10, pyruvic acid 3, picrotoxin 0.03 and DL-APV 0.03 (pH 7.35, 330 mOsm/kg, 31°C). Whole-cell voltage-clamp recordings were obtained from visually identified Purkinje cells. Patch pipets were pulled from borosilicate glass capillaries (1.5 mm OD, 0.86 mm ID; Clark Medical Instruments) and fire-polished to achieve a resistance of 3–6 MW when filled with a solution containing (in mM) K gluconate 60, K methanesulfonate 60, KCl 20, 6H2O⋅MgCl2 1.0, Na2ATP 4.0, NaGTP 0.2, HEPES 30, EGTA 10, CaCl2 0.8 and reduced glutathione 1.0 (pH 7.4, 310 mOsm/kg). The series resistance for achieving whole-cell configuration was 7–15 MW and was not compensated. To evoke EPSC from voltage-clamped Purkinje cells (–65 mV), a pipet (0.5–1 MW) containing extracellular solution was positioned above the soma of a nearby granule neuron. Extracellular stimulation (1–5 V) was delivered every 5 s via an isolation unit (SS-201J; Nihon Kohden). Recordings were made using an Axopatch 1D amplifier (Axon instruments, Foster City, CA). Signals were filtered at 5 kHz, digitized at 20 kHz (Digidata 1200) and stored in a computer. The EPSC amplitude and input resistance were continuously monitored using Pclamp 7.1 (Axon Instruments). To activate PKC, 200 nM TPA was added to the superfusion saline, for 20 min.
HEK 293 cells
cDNAs encoding full-length GluR2 and the RGS-His GRIP fragment were cloned into pcDNA 3.1 (+) and transfected into HEK 293 cells using the calcium phosphate precipitation method (Wigler et al., 1979). After 48 h, the cells were fixed with 4% paraformaldehyde followed by incubation with primary antibodies, visualized with fluorescein isothiocyanate (FITC)- or rhodamine-conjugated secondary antibodies, and analyzed by confocal microscopy (MRC-1000; Bio-Rad).
Cultured Purkinje cells
Dissociated cerebellar neuronal cultures were prepared from the brains of 20- to 21-day-old Wistar rat fetuses according to a previously reported method (Furuya et al., 1998).
Immunocytochemistry
Cells were fixed as described previously (Liao et al., 1999) (for GluR2 staining) or by incubation with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 h at 4°C (for F-actin staining), followed by washing three times with PBS. In order to stain GluR2 and synaptophysin, the cultures were incubated with a blocking solution [1% bovine serum albumin (BSA) and 10% normal goat serum in PBS] and then with primary antibodies, and visualized with rhodamine-conjugated secondary antibodies. For F-actin staining, cultures were incubated with a blocking solution containing 1% Triton X-100, and incubated with rhodamine–phalloidin (Molecular Probes). Stained cells were analyzed by fluorescence microscopy (BX60; Olympus). For computer analysis of the clusters, fluorescence images were acquired using a cooled CCD camera with 5 s exposure time. The images were processed using linear filters and quantified using IPLab imaging software (Scanalytics, Inc.) as reported previously (Hirai, 2000). The change in the clusters following various stimulations was evaluated as follows: the maximum intensity of each cluster was measured and the mean intensity of the clusters per cell was calculated. Five different Purkinje cells were analyzed and the mean ± SD of the cluster intensities was determined. Clusters (1500–2000) from five Purkinje cells were quantified.
Immunoblotting and immunoprecipitation
Cells, stimulated or unstimulated, were solubilized in lysis buffer [50 mM NaF, 15 mM Na2P2O7, 100 mM β-glycerophosphate Na2, 5 mM EGTA, 5 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 25 mM benzamidine, 10 nM okadaic acid, 2 µg/ml chymostatin, 2 µg/ml pepstatin A, 5 µg/ml leupeptin, 10 µg/ml aprotinin, 3 µg/ml antipain in 50 mM PBS]. The insoluble fraction was resuspended in the same buffer supplemented with 1% Triton X-100. The supernatants were used for subsequent immunoblotting with anti-P-Ser880 antibodies and immunoprecipitation. For immunoprecipitation, anti-GRIP antibodies (2 µg) were bound to 10 µl of protein G resin (Pharmacia) in lysis buffer. The antibody-bound protein G resin was washed three times with lysis buffer. Samples prepared were applied to the antibody-bound protein G resin and incubated for 1 h at 4°C. After washing four times with lysis buffer, SDS–PAGE sample buffer was added and incubation was carried out for 5 min at 97°C. After centrifugation, the supernatant was analyzed by immunoblotting using anti-GluR2 N-terminal antibodies and an enhanced chemiluminescence (ECL) kit (Amersham). The ECL fluorescence was quantified using an image analyzer (Basic Quantifier; Japan Bio Image, Ltd). Experiments were performed independently in triplicate.
Confocal analysis of intradendritic GluR2 immunoreactivity
Cerebellar neuronal culture was treated or not treated with TPA, and immunostained after permeabilization treatment with anti-GluR2 N-terminal antibodies. Dendrites of the Purkinje cells were scanned by confocal microscopy (MRC1000; Bio-Rad) from the lower to the upper edges at 0.5 µm intervals. The middle sections of the dendrites were selected and intensities of immunoreactive intracellular GluR2 were measured. Five different cells, totaling ∼120 µm dendrite length, were examined and the mean intensity was determined as a percentage of control samples.
Cell fractionation
Cell fractionation was carried out as described by Delft et al. (1997). Briefly, cells were homogenized in homogenation buffer (250 mM sucrose, 10 mM Tris–HCl pH 7.4, 1 mM EDTA, 1 mM PMSF, 1 mM benzamidine) by 10 passages through a 24-G needle. Cell homogenate was centrifuged at 10 000 g for 12 min at 4°C and the supernatant was collected. The supernatant was again centrifuged at 150 000 g for 30 min at 4°C and the pellet was used as the 150 000 g fraction.
Cell surface biotinylation
Cell surface biotinylation was carried out using an ECL protein biotinylation module (Amersham) in accordance with the method described by the manufacturer. Then, the GluR2 protein was immunoprecipitated using anti-GluR2 C-terminal antibodies (Chemicon) and the precipitated protein was analyzed by immunoblotting. Total GluR2 and biotinylated (cell-surface-expressed) GluR2 protein were detected by anti-GluR2 N-terminal antibodies and horseradish peroxidase (HRP)-conjugated streptavidin, respectively.
Antibodies
Polyclonal anti-GluR2 N-terminal antibodies, anti-GRIP antibodies and antibodies specific for the phosphoSer880 residue of GluR2 were generated by injecting synthetic peptides into rabbits, as reported previously (Matsuda et al., 1999). Polyclonal anti-GluR2 C-terminal antibodies were obtained from Chemicon and monoclonal anti-synaptophysin antibody from Sigma.
Acknowledgments
Acknowledgements
We would like to express our gratitude to Professor Masao Ito, in whose laboratory these experiments were performed, for his advice and encouragement. We thank Dr Takao K.Hensch for critical reading of the manuscript, Mr Takashi Torashima for expert technical assistance and the laboratory animal research center in RIKEN for their help with the generation of polyclonal antibodies.
References
- Ahn S., Ginty,D.D. and Linden,D.J. (1999) A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron, 23, 559–568. [DOI] [PubMed] [Google Scholar]
- Ajima A. and Ito,M. (1995) A unique role of protein phosphatases in cerebellar long-term depression. Neuroreport, 6, 297–300. [DOI] [PubMed] [Google Scholar]
- Bennet J.A. and Dingledine,R. (1995) Topology profile for a glutamate receptor: Three transmembrane and a channeling reentrant membrane loop. Neuron, 14, 373–384. [DOI] [PubMed] [Google Scholar]
- Bliss T.V.P. and Collingridge,G.L. (1993) Asynaptic model of memory: long-term potentiation in the hippocampus. Nature, 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Burette A., Wyszynski,M., Valtschanoff,J.G., Sheng,M. and Weinberg,R.J. (1999) Characterization of glutamate receptor interacting protein immunopositive neurons in cerebellum and cerebral cortex of albino rat. J. Comp. Neurol., 411, 601–612. [PubMed] [Google Scholar]
- Crepel F. and Krupa,M. (1988) Activation of protein kinase C induces a long-term depression of glutamate sensitivity of cerebellar Purkinje cells. An in vitro study. Brain Res., 458, 397–401. [DOI] [PubMed] [Google Scholar]
- Delft S.V., Schumacher,C., Hage,W., Verkleij,A. and van Bergen en Henegouwen,P.M.P. (1997) Association and colocalization of Eps 15 with adaptor protein-2 and clathrin. J. Cell Biol., 136, 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.A., Rhee,S.G., Billah,M.M. and Hannun,Y.A. (1991) Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J., 5, 2068–2077. [DOI] [PubMed] [Google Scholar]
- Dong H., O’Brien,R.J., Fung,E.T., Lanahan,A.A., Worley,P.F. and Huganir,R.L. (1997) GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature, 386, 279–284. [DOI] [PubMed] [Google Scholar]
- Furuya S., Makino,A. and Hirabayashi,Y. (1998) An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res. Brain Res. Protoc., 3, 192–198. [DOI] [PubMed] [Google Scholar]
- Hirai H. (2000) Clustering of δ glutamate receptor is regulated by the actin cytoskeleton in the dendritic spines of cultured rat Purkinje cells. Eur. J. Neurosci., 12, 563–570. [DOI] [PubMed] [Google Scholar]
- Hirai H., Kirsch,J., Laube,B., Betz,H. and Kuhse,J. (1996) The glycine binding site of the N-methyl-aspartate receptor subunit NR1: identification of novel determinants of co-antagonist potentiation in the extracellular M3–M4 loop region. Proc. Natl Acad. Sci. USA, 93, 6031–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Maron,C. and Heinemann,S. (1994) N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron, 13, 1331–1343. [DOI] [PubMed] [Google Scholar]
- Ito M. (1989) Long-term depression. Annu. Rev. Neurosci., 12, 85–102. [DOI] [PubMed] [Google Scholar]
- Ito M. (1990) Long-term depression in the cerebellum. Semin. Neurosci., 2, 381–390. [Google Scholar]
- Ito M. and Karachot,L. (1989) Long-term depression of quisqualate-specific glutamate receptors in Purkinje cells investigated with wedge recording from rat cerebellar slices. Neurosci. Res., 7, 168–171. [DOI] [PubMed] [Google Scholar]
- Ito M. and Karachot,L. (1990) Messengers mediating long-term desensitization in cerebellar Purkinje cells. Neuroreport, 1, 129–132. [DOI] [PubMed] [Google Scholar]
- Ito M. and Karachot,L. (1992) Protein kinases and phosphatase inhibitors mediating long-term desensitization of glutamate receptors in cerebellar Purkinje cells. Neurosci. Res., 14, 27–38. [DOI] [PubMed] [Google Scholar]
- Kano M., Kato,M., Fukunaga,K. and Konnerth,A. (1996) Ca2+-induced rebound potentiation of γ-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase. Proc. Natl Acad. Sci. USA, 93, 13351–13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Fujii,H., Gotoh,Y., Morooka,T., Shimohara,S., Nishida,E. and Hirano,T. (1999) Requirement for mitogen-activated protein kinase in cerebellar long term depression. J. Biol. Chem., 274, 13498–13502. [DOI] [PubMed] [Google Scholar]
- Liao D., Zhang,X., O’Brien,R., Ehlers,M.D. and Huganir,R.L. (1999) Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nature Neurosci., 2, 37–43. [DOI] [PubMed] [Google Scholar]
- Linden D.J. (1995) Phospholipase A2 controls the induction of short-term versus long-term depression in the cerebellar Purkinje neuron in culture. Neuron, 15, 1393–1410. [DOI] [PubMed] [Google Scholar]
- Linden D.J. (1996) A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron, 17, 483–490. [DOI] [PubMed] [Google Scholar]
- Linden D.J. and Connor,J.A. (1991) Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science, 254, 1656–1659. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Xia,H., Beattie,E.C., Carrol,R.C., Zastrow,M., Malenka R.C. and Nicoll,R.A. (1999) Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron, 24, 649–658. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Mikawa,S. and Hirai,H. (1999) Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor interacting protein. J. Neurochem., 73, 1765–1768. [DOI] [PubMed] [Google Scholar]
- McDonald J.W. and Johnston,M.V. (1990) Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res. Rev., 15, 41–70. [DOI] [PubMed] [Google Scholar]
- Mintz I.M. and Bean,B.P. (1993) Block of calcium channels in rat neurons by synthetic ω-Aga-IVA. Neuropharmacology, 32, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Regan L.J. (1991) Voltage-dependent calcium current in Purkinje cells from rat cerebellar vermis. J. Neurosci., 11, 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Stern-Bach,Y. and Stevens,C.F. (1998) The tetrameric structure of a glutamate receptor channel. Science, 280, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Shi S.-H., Hayashi,Y., Petralia,R.S., Zaman,S.H., Wenthold,R.J., Svoboda,K. and Malinow,R. (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science, 284, 1811–1816. [DOI] [PubMed] [Google Scholar]
- Thompson R.F. (1986) The neurobiology of learning and memory. Science, 233, 941–947. [DOI] [PubMed] [Google Scholar]
- Wang Y.T. and Linden,D.J. (2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron, 25, 635–647. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet,R., Sim,G.K., Wold,B., Pellicer,A., Lacy,E., Maniatis,T., Silverstein,S. and Axel,R. (1979) Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell, 16, 777–785. [DOI] [PubMed] [Google Scholar]
- Wyszynski M., Kim,E., Yang,F.-C. and Sheng,M. (1998) Biochemical and immunocytochemical characterization of GRIP, a putative AMPA receptor anchoring protein, in rat brain. Neuropharmacology, 37, 1335–1344. [DOI] [PubMed] [Google Scholar]
- Zhao H.M., Wenthold,W. and Petralia,R.S. (1998) Glutamate receptor targeting to synaptic population on Purkinje cells is developmentally regulated. J. Neurosci., 18, 5517–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]