Abstract
The contribution of host-derived growth factors to tumour growth in vivo was studied using the transplantable murine mammary carcinoma, MT1, grown in syngeneic mice. Promotion of growth of the mammary carcinoma by a factor(s) from the host was evident in experiments in which the carcinoma cells were inoculated intraperitoneally. In this environment, tumours develop as multiple solid nodules, each probably arising from an individual cell or a small cluster of cells. Tumour growth was found to occur in the peritoneal cavity following inoculation of 10(3) cells, but an inoculum of as few as ten cells grew if a leucocyte-rich exudate had first been induced. To determine which host-derived growth factors might contribute to growth of MT1, extracts of the tumour were first examined for growth factor activity. Fractionation of tumour extracts by either ion-exchange chromatography or gel filtration revealed several peaks of mitogenic activity, but none of this could be attributed to epidermal growth factor (EGF). Accordingly, an anti-EGF antibody was tested as a putative inhibitor of tumour growth as any effect of this antibody could be ascribed to removal of EGF derived from the host. The antibody was found to have potent anti-tumour activity when tested against MT1 tumours that had been inoculated into the peritoneal cavity. In contrast, the antibody had little effect on growth of the discrete tumour mass which formed when MT1 was transplanted subcutaneously. The results suggest that host-derived EGF contributes to establishment of microcolonies of MT1 carcinoma within the peritoneal cavity. This may be directly, by providing growth factors to supplement those produced by the tumour until it reaches a certain critical mass to sustain autocrine growth, or indirectly, by affecting the production of other growth-stimulatory factors or cytokines.
Full text
PDF
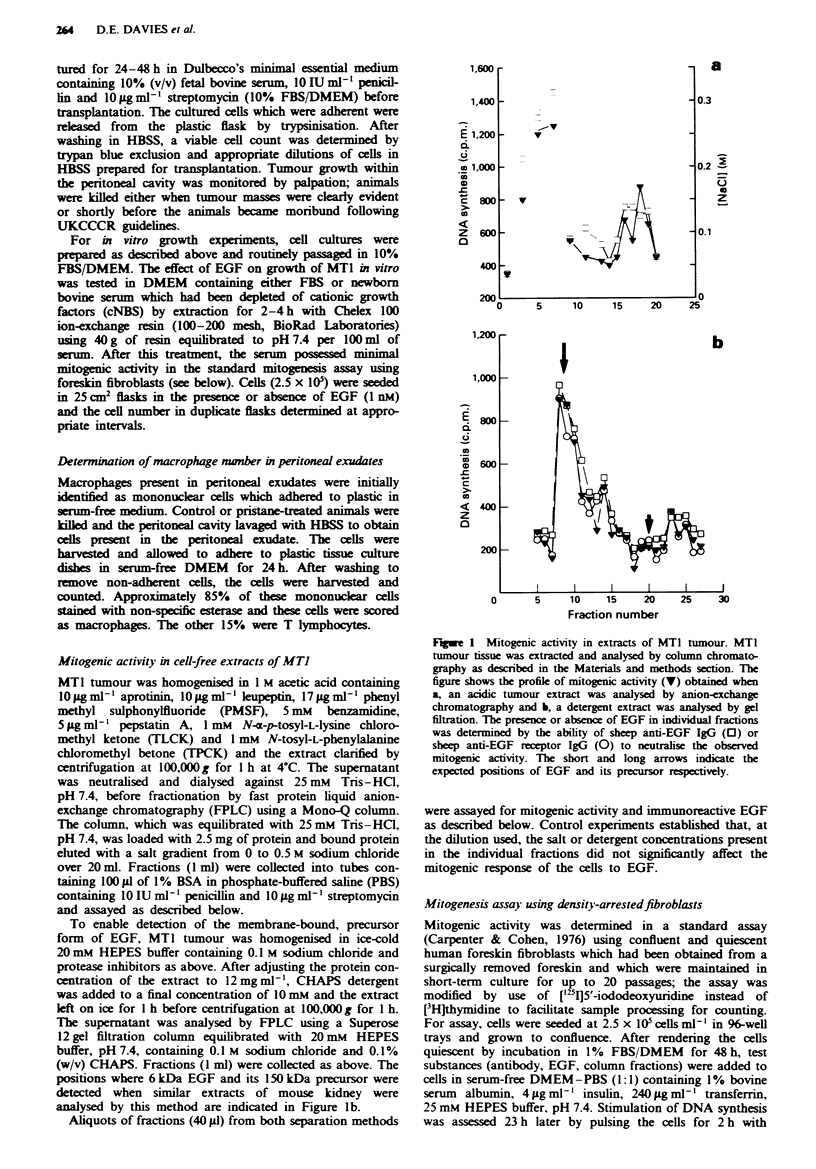

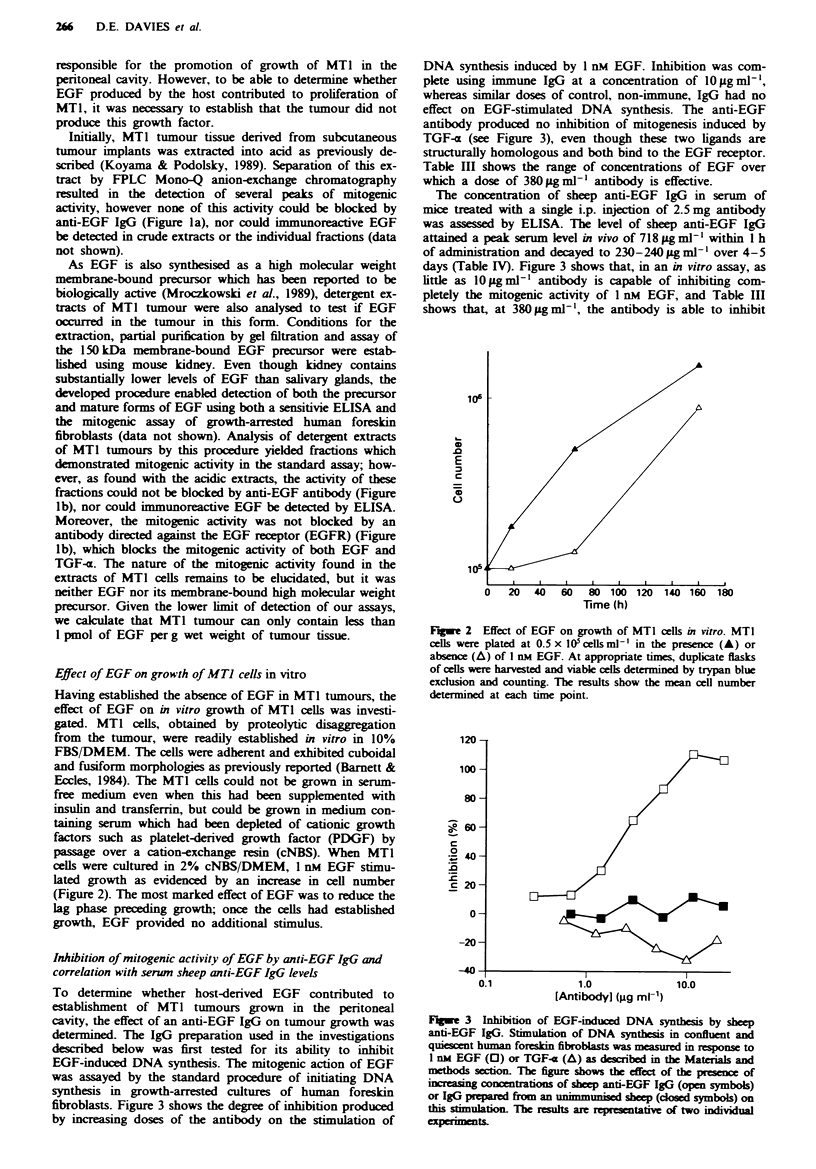



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett S. C., Eccles S. A. Studies of mammary carcinoma metastasis in a mouse model system. I: Derivation and characterization of cells with different metastatic properties during tumour progression in vivo. Clin Exp Metastasis. 1984 Jan-Mar;2(1):15–36. doi: 10.1007/BF00132304. [DOI] [PubMed] [Google Scholar]
- Baselga J., Norton L., Masui H., Pandiella A., Coplan K., Miller W. H., Jr, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993 Aug 18;85(16):1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- Breillout F., Antoine E., Lascaux V., Rolland Y., Poupon M. F. Promotion of micrometastasis proliferation in a rat rhabdomyosarcoma model by epidermal growth factor. J Natl Cancer Inst. 1989 May 3;81(9):702–705. doi: 10.1093/jnci/81.9.702. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Chemaly P., Jr Many factors contribute to racial differences in cancer experience. J Natl Cancer Inst. 1989 May 3;81(9):660–662. doi: 10.1093/jnci/81.9.660. [DOI] [PubMed] [Google Scholar]
- Divgi C. R., Welt S., Kris M., Real F. X., Yeh S. D., Gralla R., Merchant B., Schweighart S., Unger M., Larson S. M. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991 Jan 16;83(2):97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- Ginsburg E., Vonderhaar B. K. Epidermal growth factor stimulates the growth of A431 tumors in athymic mice. Cancer Lett. 1985 Sep 15;28(2):143–150. doi: 10.1016/0304-3835(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Hwang D. L., Lev-Ran A., Yen C. F., Sniecinski I. Release of different fractions of epidermal growth factor from human platelets in vitro: preferential release of 140 kDa fraction. Regul Pept. 1992 Jan 23;37(2):95–100. doi: 10.1016/0167-0115(92)90658-h. [DOI] [PubMed] [Google Scholar]
- Koyama S. Y., Podolsky D. K. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989 May;83(5):1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi H., Okamoto S., Oka T. Evidence for the involvement of the submandibular gland epidermal growth factor in mouse mammary tumorigenesis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5940–5943. doi: 10.1073/pnas.82.17.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le P. T., Lazorick S., Whichard L. P., Haynes B. F., Singer K. H. Regulation of cytokine production in the human thymus: epidermal growth factor and transforming growth factor alpha regulate mRNA levels of interleukin 1 alpha (IL-1 alpha), IL-1 beta, and IL-6 in human thymic epithelial cells at a post-transcriptional level. J Exp Med. 1991 Nov 1;174(5):1147–1157. doi: 10.1084/jem.174.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Mattila A. L., Viinikka L., Saario I., Perheentupa J. Human epidermal growth factor: renal production and absence from plasma. Regul Pept. 1988 Oct;23(1):89–93. doi: 10.1016/0167-0115(88)90424-7. [DOI] [PubMed] [Google Scholar]
- Mroczkowski B., Reich M., Chen K., Bell G. I., Cohen S. Recombinant human epidermal growth factor precursor is a glycosylated membrane protein with biological activity. Mol Cell Biol. 1989 Jul;9(7):2771–2778. doi: 10.1128/mcb.9.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P., Alexander P., Senior P. V., Fleming J., Kirkham N., Taylor I. Mechanisms of organ selective tumour growth by bloodborne cancer cells. Br J Cancer. 1988 Jan;57(1):19–31. doi: 10.1038/bjc.1988.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Kasai K., Banba N., Ishikawa M., Shimoda S. Release of human epidermal growth factor from platelets in accordance with aggregation in vitro. Endocrinol Jpn. 1989 Feb;36(1):23–28. doi: 10.1507/endocrj1954.36.23. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Hirai M., Shimizu N. Stimulation by EGF of the growth of EGF receptor-hyperproducing tumor cells in athymic mice. Int J Cancer. 1987 Nov 15;40(5):706–710. doi: 10.1002/ijc.2910400523. [DOI] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Shapiro L. J., Fisher D. A., Barajas L. In situ hybridization of prepro-epidermal growth factor mRNA in the mouse kidney. Am J Physiol. 1989 Apr;256(4 Pt 2):F632–F638. doi: 10.1152/ajprenal.1989.256.4.F632. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Singletary S. E., Frappaz D., Tucker S. L., Larry L., Brock W. A., Spitzer G. Epidermal growth factor effect on serum-free growth of primary and metastatic human tumors. Int J Cell Cloning. 1989 Jan;7(1):59–66. doi: 10.1002/stem.5530070108. [DOI] [PubMed] [Google Scholar]
- Skipper D., Jeffrey M. J., Cooper A. J., Taylor I., Alexander P. Preferential growth of bloodborne cancer cells in colonic anastomoses. Br J Cancer. 1988 Jun;57(6):564–568. doi: 10.1038/bjc.1988.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Tsutsumi O., Tsutsumi A., Oka T. Importance of epidermal growth factor in implantation and growth of mouse mammary tumor in female nude mice. Cancer Res. 1987 Sep 1;47(17):4651–4653. [PubMed] [Google Scholar]


