Abstract
The role of phosphatidylinositol 3,4,5-trisphosphate (PI3,4,5P3) and Btk in signalling by the collagen receptor glycoprotein VI was investigated. PI3,4,5P3 was increased in platelets from mice deficient in the SH2 domain-containing inositol 5-phosphatase (SHIP), in response to collagen related peptide (CRP). Tyrosine phosphorylation and activation of phospholipase Cγ2 (PLCγ2) were unaltered in SHIP–/– platelets, whereas Btk was heavily tyrosine phosphorylated under basal conditions and maximally phosphorylated by low concentrations of CRP. There was an increase in basal Ca2+, maximal expression of P-selectin, and potentiation of Ca2+ and aminophospholipid exposure to CRP in SHIP–/– platelets in the presence of Ca2+ (1 mM). Microinjection of PI3,4,5P3 into megakaryocytes caused a 3-fold increase in Ca2+ in response to CRP, which was absent in X-linked immunodeficiency (Xid) mice, which have a mutation in the PH domain of Btk. There was a corresponding partial reduction in the sustained level of intracellular Ca2+ in response to CRP in Xid mice but no change in PLC activity. These results demonstrate a novel pathway of Ca2+ entry that involves PI3,4,5P3 and Btk, and which is independent of increased PLC activity.
Keywords: Btk/phosphatidylinositol 3,4,5-trisphosphate/phospholipase C/platelets/X-linked immunodeficiency
Introduction
Collagen activates platelets through a tyrosine kinase-based signalling pathway that shares many features with signalling by immune receptors (Watson and Gibbins, 1998). Activation of this pathway is mediated through the surface receptor glycoprotein VI (GPVI), which is associated with the Fc receptor γ-chain in the membrane (Gibbins et al., 1997; Tsuji et al., 1997; Kehrel et al., 1998). GPVI was recently cloned and shown to have two extracellular immunoglobulin domains, and to have close homology to the Fcα receptor (Clemetson et al., 1999). Crosslinking of GPVI leads to tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in the Fc receptor γ-chain via the Src family kinases Fyn and Lyn (Melford et al., 1997; Ezumi et al., 1998; Briddon and Watson, 1999). This leads to binding of the protein tyrosine kinase Syk to the phosphorylated ITAM, resulting in autophosphorylation and activation. Syk plays a pivotal role in the regulation of phospholipase Cγ2 (PLCγ2), and also of the majority of other proteins that are phosphorylated downstream and/or recruited into intracellular signalling complexes upon activation of GPVI (Watson and Gibbins, 1998).
A number of the proteins that lie downstream of Syk have important roles in the regulation of PLCγ2. This includes the two adapters LAT and SLP-76, which are required for tyrosine phosphorylation and activation of the phospholipase (Gross et al., 1999a; Pasquet et al., 1999b). The p85/110 kDa heterodimeric form of phosphatidylinositol 3-kinase (PI3-kinase) is also required for activation of PLCγ2 (Pasquet et al., 1999a). This action is mediated partially through the binding of PLCγ2 to the PI3-kinase product phosphatidylinositol 3,4,5-trisphosphate (PI3,4,5P3), probably via its N-terminal PH domain by analogy to the regulation of PLCγ1 (Falasca et al., 1998). This interaction is believed to support the recruitment of PLCγ2 to the membrane (Gratacap et al., 1998; Pasquet et al., 1999a). PI3,4,5P3 is also required for the activation of a second protein with a PH domain that is involved in the regulation of PLCγ2, the tyrosine kinase Btk. Platelets deficient in Btk, isolated from donors with the X-linked immunodeficiency agammaglobulinaemia (XLA) (Tsukada et al., 1993), exhibit impaired tyrosine phosphorylation of PLCγ2 and activation upon stimulation by collagen and collagen-related peptide (CRP; Quek et al., 1998). Several other proteins expressed in platelets have PH domains and are regulated by 3-phosphorylated lipids, including the GTP exchange factor Vav (Cichowski, 1996) and protein kinase B (Franke et al., 1997). The significance of the majority of these proteins in the regulation of PLCγ2 and other events contributing to platelet activation by GPVI is not known.
The PI3-kinase pathway generates at least two second messenger lipids, PI3,4,5P3 and phosphatidylinositol 3,4-bisphosphate (PI3,4P2), which regulate a distinct set of intracellular proteins through interaction with their PH domains. PI3,4,5P3 is formed by the action of class I PI3-kinases, which includes the p85/110 kDa heterodimer described above, on phosphatidylinositol 4,5-bisphosphate. PI3,4P2 is generated either by dephosphorylation of PI3,4,5P3 (by a lipid 5-phosphatase) or through de novo synthesis by the action of a class II PI3-kinase on phosphatidylinositol 4-phosphate (Fruman et al., 1998). Platelets express at least one form of lipid 5′-phosphatase, the SH2 domain-containing inositol 5-phosphatase (SHIP) (Giurato et al., 1997). SHIP has been shown to be tyrosine phosphorylated and to undergo translocation to the cytoskeleton following outside-in signalling by the integrin GPIIb-IIIa (αIIbβ3) in human platelets (Giurato et al., 1997). The role of SHIP in platelet activation by GPVI is not known.
In order to study the relative contribution of the two lipid messengers PI3,4,5P3 and PI3,4P2 to platelet activation, we used murine platelets deficient in the lipid 5-phosphatase, SHIP (i.e. from SHIP–/– mice). An elevation of PI3,4,5P3 in the SHIP–/– platelets was associated with constitutive tyrosine phosphorylation of Btk and increased basal Ca2+ in the presence of extracellular Ca2+ ([Ca2+]e), whereas PLC activity in response to GPVI activation was unchanged. Studies on X-linked immunodeficiency (Xid) mice, which express a mutant form of Btk unable to bind PI3,4,5P3, revealed a critical role for this kinase in Ca2+ influx, which was dependent on PI3,4,5P3. This work demonstrates a novel pathway of Ca2+ entry regulated through PI3,4,5P3 and Btk but independent of an increase in PLC activity.
Results
SHIP is tyrosine phosphorylated in CRP-stimulated platelets
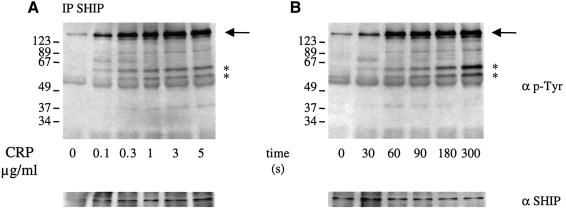
In order to investigate a possible role for SHIP in GPVI signalling in human platelets, platelets were stimulated by the GPVI-selective agonist CRP (Morton et al., 1995; Asselin et al., 1997) and the 5′-phosphatase immunoprecipitated using a specific antibody. CRP-stimulated a concentration and time-dependent increase in tyrosine phosphorylation of SHIP (Figure 1). Maximal phosphorylation was seen at a concentration of 1 µg/ml CRP after 60 s. This corresponds with the time course and concentration dependency of tyrosine phosphorylation of a large number of other proteins in CRP-stimulated platelets including Syk and PLCγ2. Several tyrosine-phosphorylated proteins were present in the SHIP immunoprecipitate and their level of tyrosine phosphorylation increased up to 300 s (Figure 1B). A tyrosine-phosphorylated band of 130 kDa migrating just below SHIP was not recognized by the SHIP antibody. Two other tyrosine-phosphorylated bands of 55 and 65 kDa were detected at 60 s and seen clearly at 300 s. A weakly phosphorylated band at 90 kDa was also detected. The identity of these bands is not known, although several could be isoforms of SHIP (Damen et al., 1998). These results demonstrate that SHIP is tyrosine phosphorylated and assembled into intracellular signalling complexes in CRP-stimulated platelets. Similar results were seen in murine platelets (not shown).
Fig. 1. SHIP is tyrosine phosphorylated in CRP-stimulated human platelets. Indomethacin-preincubated washed platelets (5 × 108/ml) were stimulated in an aggregometer cuvette with stirring in the presence of 1 mM EGTA at 37°C under the following conditions: (A) various concentrations of CRP for 2 min or (B) CRP (1 µg/ml) for varying times; (C) CRP (1 µg/ml), thrombin (1 unit/ml) and A23187 (3 µM) for 2 min in the presence of (D) BAPTA-AM (20 µM); and (E) PP1 (10 µM). The reaction was stopped by addition of ice-cold lysis buffer. Anti-SHIP immunoprecipitates were run on SDS–PAGE and transferred to PVDF membranes. Membranes were probed for phosphotyrosine using mAb 4G10 antibody (upper panel) and SHIP (lower panel). Molecular weight markers are indicated on the left. SHIP is shown by an arrow and major associated proteins by asterisks on the right. Results are representative of three to five experiments.
The G protein-coupled receptor agonist thrombin and the Ca2+ ionophore A23187 stimulate a similar level of SHIP tyrosine phosphorylation and increase in association with intracellular proteins to that induced by CRP. These experiments were performed under conditions in which activation of the integrin αΙIbβ3 was prevented by inclusion of EGTA (Figure 1). Tyrosine phosphorylation of SHIP induced either by CRP or thrombin was substantially inhibited by the chelator of intracellular Ca2+ ([Ca2+]i) BAPTA (loaded as BAPTA-AM) (Figure 1D). Src kinase inhibition by PP1 fully abolished CRP-induced SHIP tyrosine phosphorylation whereas thrombin still induced significant phosphorylation (Figure 1E). These results demonstrate that CRP and thrombin stimulate tyrosine phosphorylation of SHIP downstream of PLC through a Ca2+-dependent mechanism.
Phosphatidylinositol 3,4,5-trisphosphate is increased in SHIP–/– platelets in response to CRP
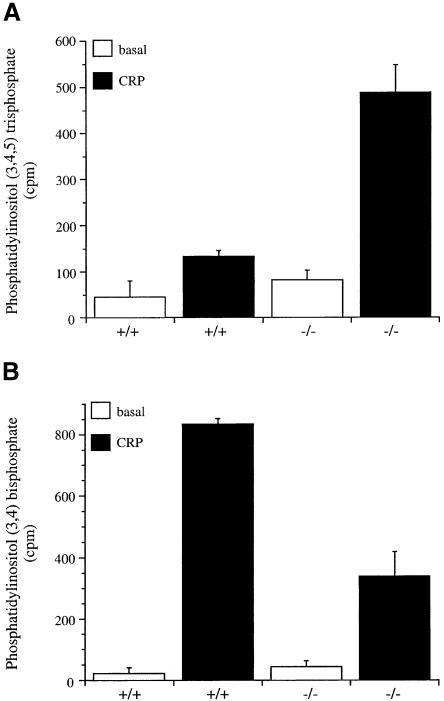
CRP has been reported to stimulate the rapid formation of [32P]PI3,4,5P3 in human platelets, peaking at 60 s after stimulation (Pasquet et al., 1999a). As shown in Figure 2A, CRP also induces formation of [32P]PI3,4,5P3 in mouse platelets (Figure 2A). In SHIP–/– platelets, the basal level of [32P]PI3,4,5P3 was slightly elevated and the response to CRP increased ∼5-fold relative to SHIP+/+ platelets, whereas the level of [32P]PI3,4P2 was reduced by ∼60% (Figure 2A and B). In comparison, the level of [32P]phosphatidylinositol 4,5-bisphosphate was similar in SHIP+/+ and SHIP–/– platelets (not shown). These results demonstrate a role for SHIP in the metabolism of PI3,4,5P3 and reveal that PI3,4P2 is formed, at least partially, by metabolism of PI3,4,5P3. The SHIP-independent increase in PI3,4P2 could be derived from either PI3,4,5P3 by the action of another lipid 5′-phosphatase, such as SHIP2 (Pesesse et al., 1998) (it is not known whether SHIP2 is expressed in platelets), or from phosphatidylinositol 4-phosphate by the action of a class II PI3-kinase (Zhang et al., 1998).
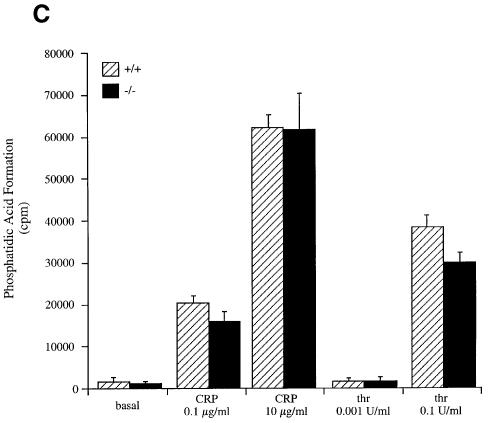
Fig. 2. Phospholipid metabolism in SHIP–/– platelet mice in response to CRP. Platelets (4 × 108/ml) were labelled with 1 mCi/ml [32P]orthophosphate (A–C) or loaded with Fura-2-AM (D). Stimulation was performed at 37°C for 2 min with: 0.1 µg/ml CRP (A) and (B); 0.1 and 10 µg/ml CRP and 0.001 and 0.1 U/ml thrombin (C); and 5 µg/ml CRP (D). In (A–C), the reaction was stopped by addition of 50 µl of 5 N HCl. After phospholipid extraction and deacylation as described in Materials and methods, samples were run on HPLC. Inositol lipids were identified by comparison with standards. PI3,4,5P3 (A); PI3,4P2 (B); phosphatidic acid (PA) (C); phosphatidic acid formation was analysed after phospholipid extraction by TLC and quantification by scintillation counting. (D) Ca2+: Ca2+ was measured in Fura-2-loaded platelets by recording fluorescence at 510 nm following excitation at 340 and 380 nM. Results in (A) and (B) are expressed as a mean ± range. Similar results were obtained in two separate experiments. Results in (C) are from one experiment carried out in triplicate and is representative of three. Results in (D) are from six experiments and are shown as mean ± SE.
Phospholipase C activity is not changed in SHIP–/– platelets
In order to determine whether PLC activity is altered in the SHIP–/– platelets, we measured the 1,2-diacylglycerol metabolite, phosphatidic acid, and the major protein kinase C substrate, pleckstrin. Although phosphatidic acid can be derived by phospholipase D activity, the level of this enzyme in platelets is low and the product would not be significantly radiolabelled under the non-equilibrium conditions used (Huang et al., 1991). The level of [32P]phosphatidic acid was similar in SHIP+/+ and SHIP–/– platelets under basal conditions and in response to stimulation by low and high concentrations of CRP and thrombin (Figure 2C). Similar results were seen for [32P]phosphorylation of pleckstrin (not shown). These results demonstrate that PLC activation is not enhanced in response to CRP and thrombin in the SHIP–/– platelets.
A further indirect marker of PLC activity is the mobilization of Ca2+. This was measured in Fura-2-loaded platelets in Ca2+-free medium containing EGTA (estimated [Ca2+]e <1 nM). The basal level of Ca2+ and increases in response to CRP were similar in SHIP+/+ and SHIP–/– platelets consistent with a lack of change in PLC activity (not shown). In contrast, there was a 2-fold increase in the basal concentration of Ca2+ in the presence of 1 mM [Ca2+]e in the SHIP–/– platelets relative to controls (Table I and Figure 2D). [Ca2+]i was also increased in response to CRP in the SHIP–/– platelets in the presence of [Ca2+]e, although this was primarily the result of the increase in the basal level (Table I and Figure 2D). This suggests a role of 3-phosphorylated lipids in regulating Ca2+ influx in platelets.
Table I. Change in intracellular Ca2+ induced by CRP in SHIP–/– mice platelets.
| SHIP genotype | Basal | CRP |
|---|---|---|
| +/+ | 98 ± 9 (n = 6) | 365 ± 26 (n = 5) |
| –/– | 177 ± 11 (n = 8)* | 500 ± 31 (n = 6)* |
Fura-2 loaded platelets (108/ml) were stimulated in a spectrofluorimeter cuvette at 37°C under stirring in the presence of 1 mM extracellular Ca2+. Baseline was recorded for 30 s and then CRP (10 µg/ml) was added. The asterisk indicates that p <0.05 when values are compared with the control data.
Btk is hyperphosphorylated in SHIP–/– platelets
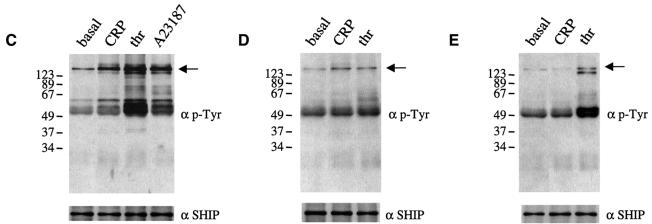
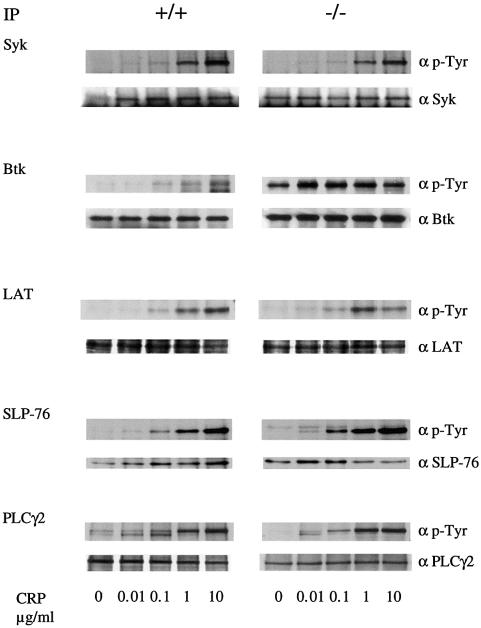
The general pattern of tyrosine phosphorylation in control and SHIP–/– platelets in response to CRP was similar, with the exception of a broad 75 kDa band that comigrates with Syk, Btk and SLP-76 (not shown). An increase in phosphorylation of this band was also seen in non-stimulated SHIP–/– platelets. Phosphorylation of individual proteins implicated in the regulation of PLCγ2, notably those with a molecular weight of 70–76 kDa, was investigated in the SHIP–/– mice following immunoprecipitation using specific antibodies. CRP stimulated tyrosine phosphorylation of Btk, SLP-76 and Syk in SHIP+/+ platelets over the concentration range 0.1–10 µg/ml (Figure 3). Tyrosine phosphorylation of Syk, LAT and PLCγ2 was not significantly modified in the SHIP–/– platelets compared with SHIP+/+ cells (Figure 3). However, there was a slight increase in tyrosine phosphorylation of SLP-76 and a marked increase in phosphorylation of Btk in the SHIP–/– platelets (Figure 3). Btk was hyperphosphorylated in unstimulated platelets and maximally phosphorylated by the lowest concentration of CRP (0.01 µg/ml) that was used (Figure 3). Btk is therefore responsible for the increase in tyrosine phosphorylation of the 75 kDa band observed in whole-cell lysates.
Fig. 3. BTK is hyperphosphorylated in SHIP–/– mice platelets. Washed platelets (5 × 108/ml) were stimulated by the indicated CRP concentration for 2 min in the presence of 1 mM EGTA and the reaction was stopped by addition of ice-cold lysis buffer. After immunoprecipitation, samples were run on SDS–PAGE and transferred to PVDF membrane. Membranes were probed for phosphotyrosine using mAb 4G10 (upper panel) and the immunoprecipitated proteins (lower panel). Results are representative of three experiments.
The increase in tyrosine phosphorylation of Btk in the presence of an elevated level of PI3,4,5P3 is consistent with observations in other cells. PI3,4,5P3 binds to the PH domain of Btk supporting its association to the membrane where it is phosphorylated by a Src family kinase (Mahajan et al., 1995; Afar et al., 1996; Rawlings et al., 1996; Li et al., 1997; Scharenberg et al., 1998). Btk activated in this way undergoes autophosphorylation and stimulates phosphorylation of cellular substrates.
Measurement of P-selectin expression and procoagulant activity in SHIP–/– mice
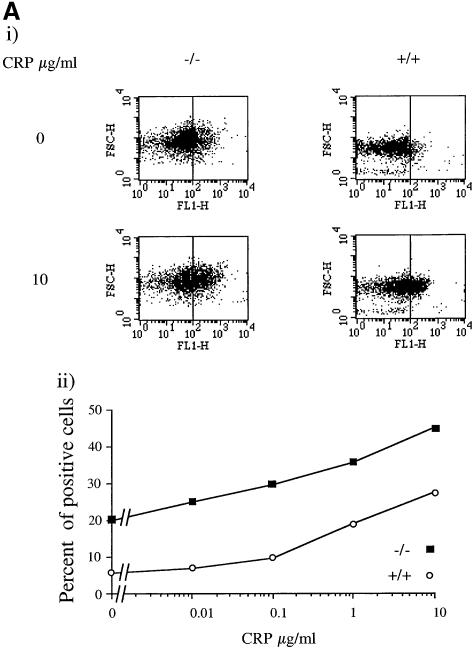
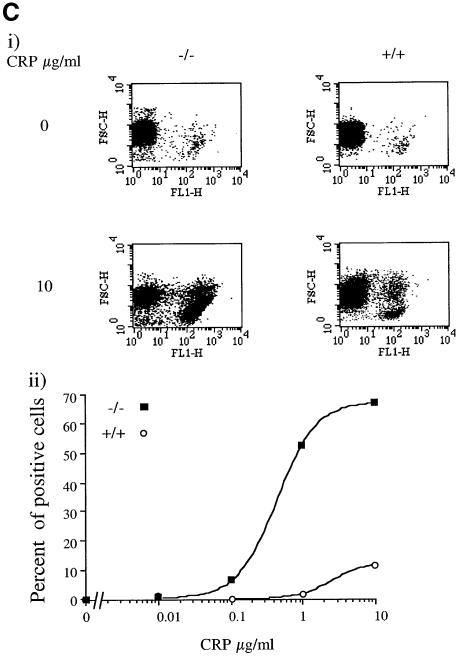
The functional consequence of the increase in PI3,4,5P3 in response to CRP was investigated by measurement of P-selectin expression and aminophospholipid exposure (procoagulant activity). P-selectin is a marker of α-granule secretion, which can be monitored by flow cytometry using a specific antibody. The exposure of aminophospholipids can also be measured by flow cytometry using annexin V, which binds with high affinity to phosphatidylserine. Aminophospholipid exposure is only seen in Ca2+-containing medium because of the high intracellular levels of the cation required for this response (Dachary-Prigent et al., 1993). Expression of P-selectin is not dependent on [Ca2+]e, but is increased in its presence (compare Figure 4A and B).
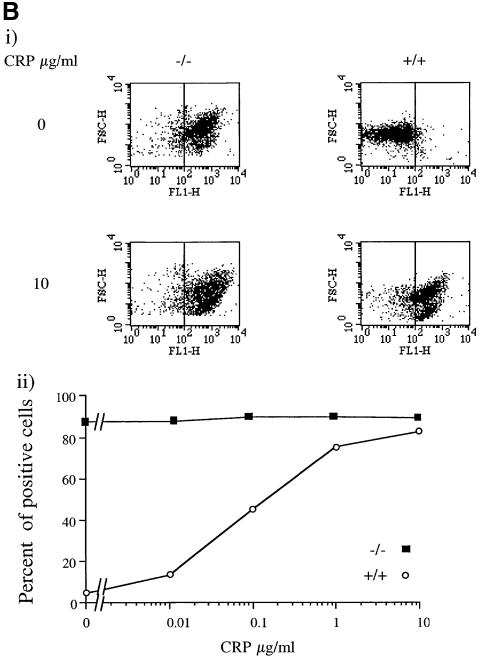
Fig. 4. Ca2+-dependent potentiation of P-selectin expression and procoagulant activity in SHIP–/– mice platelets. Washed platelets (5 × 108/ml) were stimulated with the indicated CRP concentration for 2 min in the presence of 1 mM EGTA (A) or 1 mM Ca2+ (B) and (C). Aliquots were incubated with P-selectin antibody and FITC-conjugated secondary antibodies for 10 min (A and B) or 10 µl of annexin-V–FITC (C). Results are presented as a dot-plot, showing fluorescence versus size (FL1/FSC), or expressed as a percentage of total cells responding (lower panel). Results are from one experiment that is representative of five separate experiments.
A significantly greater number of cells (∼20%) show expression of P-selectin in SHIP–/– platelets in the absence of [Ca2+]e (free Ca2+ <1 nM) compared with controls (∼5%), suggesting that a significant degree of platelet activation has occurred either during platelet preparation or in vivo (Figure 4A). Taking the higher base line into account, the concentration–response relationship for P-selectin expression by CRP is similar in the SHIP–/– and control platelets in Ca2+-free medium, i.e. the EC50 value, and the increases in magnitude of expression in response to CRP were not significantly different in the two groups (Figure 4A). In the presence of [Ca2+]e (1 mM), there was a dramatic increase in expression of P-selectin under basal conditions, with the level of expression being higher than that for the peak response to CRP in Ca2+-free medium (Figure 4B). In contrast, the basal level of P-selectin expression was not altered in SHIP+/+ platelets in the presence of [Ca2+]e (1 mM) (Figure 4B). It seems likely that the increase in [Ca2+]i observed under basal conditions in the SHIP–/– platelets is sufficient to cause P-selectin expression in the presence of 1 mM [Ca2+]e.
There was no significant change in expression of aminophospholipids in control and SHIP–/– platelets in the presence of [Ca2+]e, reflecting a need for a much higher level of [Ca2+]i to activate this pathway than for exposure of P-selectin. Exposure of aminophospholipids in response to CRP was markedly potentiated in the SHIP–/– platelets in the presence of Ca2+, consistent with the increase in influx of the cation. The number of cells that stained positively for aminophospholipids in response to a maximally effective concentration of CRP was increased from 10 to 70% (Figure 4C). This was accompanied by a 10-fold reduction in the EC50 value for CRP (Figure 4C).
Ca2+ entry is reduced in platelets and megakaryocytes from Xid mice
The above observations demonstrate that elevation of PI3,4,5P3 leads to a dramatic increase in tyrosine phosphorylation of Btk and increase in basal Ca2+ in the presence of [Ca2+]e. It is therefore of interest that several groups have proposed a role for Btk in the regulation of the sustained phase of Ca2+ influx in B lymphocytes following activation of the B-cell antigen receptor (Fluckiger et al., 1998; Scharenberg et al., 1998). In order to investigate whether the increases in PI3,4,5P3, Btk phosphorylation and Ca2+ are linked, experiments were performed on Xid mice that have a mutation in the PH domain of Btk (R28C) that reduces its affinity for PI3,4,5P3 (Salim et al., 1996; Rameh et al., 1997).
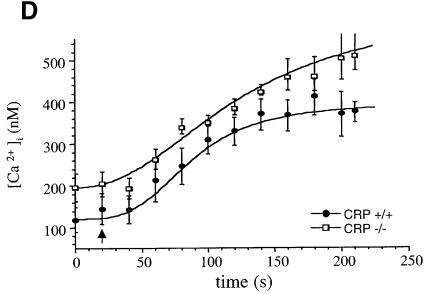
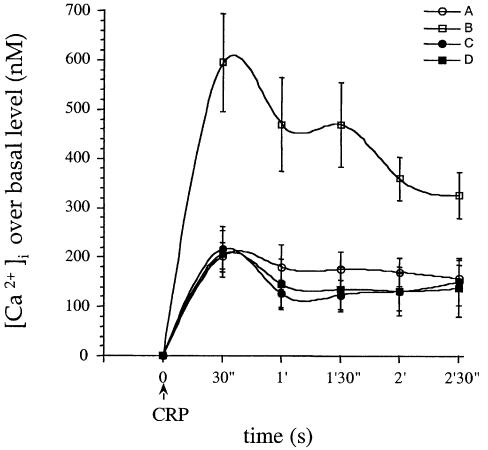
Experiments were performed in megakaryocytes in order to take advantage of their much greater size relative to platelets. This enabled us to introduce PI3,4,5P3 into the megakaryocyte by microinjection and to study its action on the response to CRP. PI3,4,5P3 had no effect on basal Ca2+ but caused a marked increase in magnitude (>3-fold) and duration (>10 min) of the Ca2+ response to CRP in control cells in the presence of 200 µM [Ca2+]e (Figure 5). In contrast, there was no potentiation of the Ca2+ response to CRP following microinjection of PI3,4,5P3 into Xid megakaryocytes, demonstrating a critical role for the PH domain of Btk in this response (Figure 5). These results provide evidence for a pathway of regulation of Ca2+ influx regulated through PI3,4,5P3 and Btk.
Fig. 5. PI3,4,5P3 potentiates Ca2+ influx in response to CRP in control but not Xid megakaryocytes. Megakaryocytes from Balb/c (A and B) or Xid (C and D) mice were microinjected with Fura-2 (A and C) or Fura-2 and PI3,4,5P3 (B and D) and the changes in [Ca2+]i has been determined in response to CRP 4 µg/ml. [Ca2+]e was 200 µM. Time 0 was set when CRP was added to the megakaryocytes, and the calcium response was then calculated at each 30 s after CRP addition up to 150 s. Each curve represents results of eight to 10 cells from a minimum of four mice.
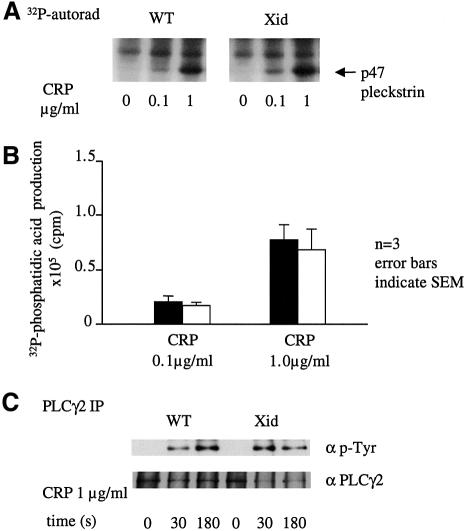
The physiological significance of this pathway can be addressed by comparison of the increase in [Ca2+]i in control and Xid platelets and megakaryocytes in response to CRP. It is possible to perform this study because activation of PLCγ2 in response to GPVI is not altered significantly in Xid mice in response to CRP. This is illustrated by the similar degree of phosphorylation of pleckstrin, formation of phosphatidic acid and tyrosine phosphorylation of PLCγ2 in response to CRP in control and Xid platelets (Figure 6). This contrasts with studies on XLA human platelets where a marked inhibition of PLCγ2 phosphorylation and activation is seen (Quek et al., 1998).
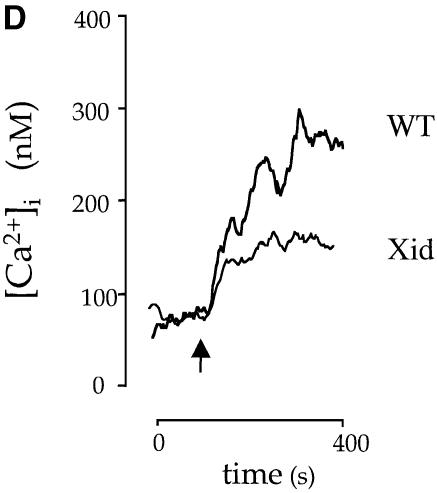
Fig. 6. PLC activity is unchanged but the sustained phase of Ca2+ entry is reduced in Xid platelets in response to CRP. (A) Pleckstrin phosphorylation. 32P-labelled platelets were stimulated by CRP for 2 min and the reaction was stopped by addition of sample buffer. Following electrophoresis, the gel was dried and pleckstrin phosphorylation was analysed by autoradiography. (B) Phosphatidic acid. 32P-labelled platelets were stimulated by CRP for 2 min and the reaction was stopped by addition of chloroform:methanol (1:2) and phosphatidic acid separated by thin layer chromatography as described in Materials and methods. Following autoradiography, spots were scraped and counted by Cerenkov. Results are the mean of three experiments. (C) Control and Xid platelets were stimulated with CRP (2 µg/ml) and then PLCγ2 was immunoprecipitated. PLCγ2 tyrosine phosphorylation was detected in the 4G10 blot (upper panel) and PLCγ2 loading was detected by reprobing (lower panel). (D) Platelets from control and Xid mice were loaded with Fura-2 as described in the Materials and methods. The baseline was recorded for 30 s and platelets were stimulated with CRP (indicated by an arrow) in the presence of [Ca2+]e (1 mM). The concentration of [Ca2+]i is calculated using the ratio of fluorescence, using a Kd of 224 nM. The trace is representative of 10 experiments from five animals.
In the presence of [Ca2+]e, CRP stimulated a rapid initial peak in [Ca2+]i that decayed to a plateau in control platelets and megakaryocytes (Figure 6D). A similar initial peak in [Ca2+]i was observed in platelets and megakaryocytes from Xid mice in response to CRP, whereas the plateau was reduced by 20–40% in both cases (Figures 5 and 6). The latter supports a role for Btk in the sustained entry of Ca2+. The plateau was abolished in the absence of [Ca2+]e (not shown) demonstrating the presence of another mechanism of Ca2+ influx, which is presumably regulated by capacitative Ca2+ entry.
Discussion
In the present study, the role of the inositol 5′-phosphatase SHIP and the PH domain of the tyrosine kinase Btk have been investigated in platelets through respective studies on SHIP–/– and Xid mice stimulated by the collagen receptor GPVI. In response to CRP, the level of PI3,4,5P3 is elevated, whereas that of PI3,4P2 is reduced in SHIP–/– platelets demonstrating a major role for SHIP in the metabolism of PI3,4,5P3. These changes were associated with near maximal tyrosine phosphorylation of Btk under basal conditions, whereas PLC activity was unchanged. There was also an increase of basal Ca2+ in the SHIP–/– platelets in the presence of [Ca2+]e, and potentiation of Ca2+ influx in response to CRP, although this appeared to be primarily the result of the increase in basal Ca2+. The increase in phosphorylation of Btk is likely to be mediated by recruitment to the plasma membrane through interaction with PI3,4,5P3 and subsequent phosphorylation by a Src family kinase, probably Lyn (Mahajan et al., 1995; Afar et al., 1996; Rawlings et al., 1996; Li et al., 1997; Scharenberg et al., 1998). The increase of [Ca2+]i in SHIP–/– platelets provides a molecular explanation for the dramatic expression of P-selectin under basal conditions, and potentiation of aminophospholipid exposure by CRP in Ca2+-containing buffer. A lower level of [Ca2+]i is required for expression of P-selectin, accounting for the qualitative difference in results for the two responses.
These results are similar to observations in bone marrow-derived mast cells and chicken DT-40 deficient in SHIP. An increase in Ca2+ influx was observed upon agonist stimulation that was not associated with a change in PLCγ activity as demonstrated by measurement of IP3 (Bolland et al., 1998; Huber et al., 1998b). In contrast to these studies, however, the major effect in the SHIP–/– platelets was on the basal level of Ca2+ in the presence of [Ca2+]e. This suggests that the 3-phosphorylated lipid-dependent pathway of Ca2+ influx is near maximal under basal conditions in the SHIP–/– platelets. This is consistent with the observation that tyrosine phosphorylation of Btk is also near maximal under basal conditions in view of its proposed role in Ca2+ influx (see later).
Subsequent studies in megakaryocytes were designed to address whether the increase in Ca2+ was mediated by PI3,4,5P3. Microinjection of PI3,4,5P3 in control megakaryocytes had no effect on basal Ca2+ but potentiated Ca2+ influx in response to CRP. This observation provides strong evidence for a role of PI3,4,5P3 in the regulation of [Ca2+]i by GPVI, presumably mediated by recruitment of Btk or PLCγ2 to the membrane. The lack of effect of microinjected PI3,4,5P3 on basal Ca2+ in the megakaryocytes is in contrast to the increase observed in the SHIP–/– platelets. This may reflect critical differences in experimental conditions between the two studies. For example, PI3,4,5P3 is unable to cause rapid translocation of Btk to the plasma membrane (Peter Cullen, personal communication) and yet, in the SHIP–/– platelets, there is near maximal tyrosine phosphorylation of Btk under basal conditions. This suggests that prolonged elevation of PI3,4,5P3 has caused a slow recruitment of the tyrosine kinase to the membrane for phosphorylation by a Src family kinase.
Studies on Xid mice provide evidence that the effect of PI3,4,5P3 requires an intact PH domain in Btk for increased Ca2+ influx, revealing a critical role for the kinase in this pathway. The data therefore support a model in which PI3,4,5P3 regulates Ca2+ entry through Btk. A partial reduction in the plateau phase of Ca2+ elevation was also observed in Xid platelets and megakaryocytes in response to CRP, suggesting that the PI3,4,5P3/Btk pathway has a small role in regulating Ca2+ influx upon GPVI activation under physiological conditions. A similar conclusion was made for the regulation of Ca2+ influx by PI3,4,5P3 following stimulation of the FcεR1 in bone marrow-derived mast cells (Huber et al., 1998b). Capacitative Ca2+ entry is likely to be the major pathway regulating influx following depletion of intracellular Ca2+ stores.
The mechanism through which PI3,4,5P3 and Btk regulate Ca2+ entry in platelets/megakaryocytes remains to be clarified. However, the observation that PLCγ2 phosphorylation and activity are unchanged in the SHIP–/– platelets in response to CRP demonstrates that the increase does not occur at the level of PLC. This is in accordance with reports in SHIP–/–-deficient mast cells and chicken DT 40 cells where PLC activity was also unchanged (Bolland et al., 1998; Huber et al., 1998b). The results contrast with the situation in B lymphocytes where Btk has been shown to play a role in the activation of PLCγ2 leading to potentiation of capacitative Ca2+ entry following stimulation of the B-cell receptor (Fluckiger et al., 1998; Scharenberg and Kinet, 1998). The PI3,4,5P3–Btk pathway in the platelet may account for the recent observation that PI3,4,5P3 regulates Ca2+ entry in rabbit platelets (Lu et al., 1998).
The increase in tyrosine phosphorylation of Btk in the SHIP–/– platelets did not lead to an increase in tyrosine phosphorylation of Syk, Lat and PLCγ2, suggesting that none of these are major substrates for the Tec family kinase. This is particularly surprising for PLCγ2 since it is a recognized substrate of Btk (Scharenberg et al., 1998). Moreover, phosphorylation of PLCγ2 is reduced in XLA human platelets stimulated through GPVI (Quek et al., 1998) and abolished in Btk-deficient chick B lymphocytes stimulated via the B-cell antigen receptor (Takata and Kurosaki, 1996). This may reflect an important difference in the role of Btk between species. Alternatively, phosphorylated Btk may not be a rate-limiting factor in mediating activation of PLCγ2 or may not have access to the phospholipase in SHIP–/– platelets. The small increase in tyrosine phosphorylation of SLP-76 suggests that Btk may play a minor role in regulating its phosphorylation, whereas Syk is believed to play a much greater role (Gross et al., 1999a).
The similar level of PLC activity in SHIP+/+ and SHIP–/– platelets under basal conditions and following stimulation by CRP was also unexpected given the role of the PI3,4,5P3 in the regulation of PLCγ2 by GPVI and also by FcγRIIA (Gratacap et al., 1998; Pasquet et al., 1999a). This work therefore demonstrates that elevation of PI3,4,5P3 is insufficient to activate PLCγ2 or to potentiate its activation by CRP. Similar conclusions were reached in bone marrow-derived mast cell from SHIP-deficient mice stimulated by steel factor (Huber et al., 1998b). It seems that only a fraction of the generated PI3,4,5P3 is required for regulation of PLCγ2 in response to CRP, and this may explain why near-maximal concentrations of the PI3-kinase inhibitors wortmannin and LY294002 are required to inhibit PLC activation by CRP in platelets (Pasquet et al., 1999a).
In conclusion, our results show that SHIP is involved in the regulation of PI3,4,5P3 metabolism in platelets and that elevation of the 3-phosphorylated lipid is correlated with activation of Btk and increased Ca2+ influx following activation of GPVI, and that this is independent of PLC. This novel pathway of Ca2+ entry accounts for up to 30% of the sustained influx response under physiological conditions, but may play a much greater role in diseased states that lead to elevation of PI3,4,5P3 or activation of Btk. The combined roles of PI3-kinase and Btk in Ca2+ influx may explain the similar phenotypes of mice deficient in functional Btk and the p85α subunit of PI3-kinase (Fruman et al., 1999; Suzuki et al., 1999).
Materials and methods
Materials
Rabbit anti-Syk, anti-Btk and anti-PLCγ2 were a generous gift from Dr M.G.Tomlinson (DNAX, Palo Alto, CA). Anti-SHIP antibody was a kind gift from Dr Peter Parker and Rudiger Woscholski (Giurato et al., 1997). Rabbit anti-SLP-76 was a kind gift from Dr Gary Koretzky (University of Pennsylvania, PA). Phosphatidylinositol 1-stearoyl; 2-arachidonyl 3,4,5-trisphosphate (PI3,4,5P3) was from Alexis Incorporation (Nottingham, UK). The rat P-selectin antibody was from Pharmingen (Becton Dickinson, Oxford, UK). All other reagents were of analytical grade or from previously described sources (Gross et al., 1999b; Pasquet et al., 1999b). A breeding pair of Xid mice (Berning et al., 1980) on a CBA/N background were kindly donated by Dr V.Tybulewicz (National Institutes of Mill Hill, London) and bred in the Department of Pharmacology. SHIP–/– mice (Helgason et al., 1998) were bred in the British Columbia Cancer Research Centre, Vancouver, Canada.
Preparation of platelets
Human blood was donated by volunteers who had denied taking aspirin in the 10 days prior to the experiment. Murine blood was taken by cardiac puncture following CO2 narcosis. Platelets were isolated from platelet-rich plasma by centrifugation at 1000 g for 10 min in the presence of prostacyclin (0.1 µg/ml) and resuspended in a modified Tyrode’s–HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3) in the presence of the cyclo-oxygenase inhibitor indomethacin (10 µM). Human platelets were centrifuged an additional time at 1000 g for 10 min following addition of prostacyclin (0.1 µg/ml) and resuspended at a concentration of 5 × 108 cells/ml in the above buffer. Ca2+ (0.2–1 mM) or EGTA (1 mM ‘Ca2+ free buffer’) were added as required. Experiments were performed at 37°C in an aggregometer with stirring at 1200 r.p.m. unless stated.
Platelet labeling and phosphoinositides analysis
Platelets suspended in phosphate-free Tyrode’s–HEPES buffer were incubated with 1 mCi/ml of [32P]orthophosphate for 1 h at 37°C. After two centrifugations at 1000 g, platelets were resuspended at 5 × 108/ml in Tyrode’s–HEPES buffer plus 10 µM indomethacin and left for 15 min. Reactions were stopped by addition of an equal volume of 1 N HCl, and phosphoinositides were analysed by HPLC after deacylation as described (Huber et al., 1998b). [32P]phosphatidic acid was detected by thin layer chromatography (TLC) and quantified by counting (Gratacap et al., 1998).
Immunoprecipitation and immunoblotting
Reactions were stopped by adding an equal volume of ice-cold lysis buffer [20 mM Tris, 300 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2% (v/v) NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 µg/ml pepstatin A, pH 7.3]. Non-lysed cells and debris were removed by centrifugation and lysates were precleared by mixing with protein A–Sepharose for 1 h at 4°C. Proteins were precipitated as described previously (Pasquet et al., 1999b), separated by SDS–PAGE and transferred to polyvinylidene difluoride membranes. Blots were developed using an enhanced chemiluminescence (ECL) detection system.
Flow cytometry
In the standard assay, samples (5 × 106 platelets) in Tyrode’s–HEPES buffer were incubated with annexin V–FITC (10 µl) or P-selectin antibody and secondary FITC antibody (5 µl each) for 10 min at 22°C. After a 5-fold dilution in Tyrode’s–HEPES buffer, samples were analysed using a Becton Dickinson FACScalibur flow cytometer. For annexin-V binding all steps were carried out in Tyrode’s–HEPES buffer containing 2 mM CaCl2. Ten thousand events were acquired from each sample in both cases. The light scatter and the fluorescence signals were set in logarithmic gain. Results were analysed as dot-plots of forward scatter (FSC) versus fluorescence intensity channel 1 (FL1) and normalized as a percentage of total cells responding. Positive cells both for P-selectin and annexin-V were selected from unstimulated platelets as described (Dachary-Prigent et al., 1993).
Measurement of cytosolic Ca2+ in platelets
Platelets isolated from platelet-rich plasma were resuspended in Tyrode’s–HEPES buffer and incubated with Fura-2-AM (2 µM) for 45 min at 37°C. Following centrifugation, platelets were resuspended at 5 × 108/ml and prewarmed to 37°C for 10 min. Stimulations were performed at a platelet concentration of 1 × 108/ml in a Perkin-Elmer LS50B spectrofluorometer at 37°C with agitation in the presence of 0.1 mM EGTA or 1 mM Ca2+. Excitation was at 340 and 380 nM, and emission at 510 nM. The concentration of [Ca2+]i was calculated using the software FLWinlab (Perkin-Elmer).
Measurement of cytosolic Ca2+ in megakaryocytes
Megakaryocytes were dissected from mouse bone marrow and used as described previously (Melford et al., 1997; Pasquet et al., 1999a). All experiments were carried out with [Ca2+]e = 200 µM at 22 ± 2°C. Fura-2 pentapotassium salt was dissolved in standard intracellular buffer (125 mM potassium glutamate, 1 mM MgCl2, 50 µM EGTA, 5 mM HEPES pH 7.2) and was present in the injection needle at a concentration of 2.5 mM, resulting in an estimated intracellular concentration of ≤200 µM. In some studies, PI3,4,5P3 was also present in the injection needle at a concentration of 1.5 µM) giving an estimated intracellular concentration of ≤150 nM. Data are presented as the peak value of [Ca2+]i after agonist addition.
Analysis of data
Results are shown as mean ± SE of at least three experiments, with the exception of the phosphoinositides studies. Differences were analysed by an unpaired Student’s t-test. In each case p <0.05 was taken as the minimum value to indicate statistical significance.
Acknowledgments
Acknowledgements
We are grateful to Drs Barnes, Farndale and Knight for the gift of CRP, and to Dr Peter Parker and Rudiger Woscholski for the antibody to SHIP. During the course of this work, J.-M.P. was supported sequentially by the Fondation pour la Recherche Médical, l’Institut National de la Santé et de la Recherche Médical and the Wellcome Trust. The work was supported by the Wellcome Trust, the British Heart Foundation (BHF) and the Heart and Stroke Foundation of British Columbia and Yukon. S.P.W. is a British Heart Foundation Senior Research Fellow. L.Q. held a BHF Studentship.
References
- Afar D., Park,H., Howell,B.W., Rawlings,D.J., Cooper,J. and Witte,O.N. (1996) Regulation of Btk by Src family tyrosine kinases. Mol. Cell. Biol., 16, 3465–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin J., Gibbins,J.M., Achison,M., Lee,Y.-H., Morton,L.F., Farndale,R.W., Barnes,M.J. and Watson,S.P. (1997) A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1. Blood, 89, 1235–1242. [PubMed] [Google Scholar]
- Berning A.K., Eicher,E.M., Paul,W.E. and Scher,I. (1980) Mapping of the X-linked immune deficiency mutation (xid) of CBA/N mice. J. Immunol., 124, 1875–1877. [PubMed] [Google Scholar]
- Bolland S., Pearse,R.N., Kurosaki,T. and Ravetch,J.V. (1998) SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity, 8, 509–516. [DOI] [PubMed] [Google Scholar]
- Briddon S.J. and Watson,S.P. (1999) Evidence for the involvement of p59fyn and p53/56lyn in signalling via the collagen receptor in human platelets. Biochem. J., 338, 203–209. [PMC free article] [PubMed] [Google Scholar]
- Cichowski K., Brugge,J.S. and Brass,L.F. (1996) Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav in platelets. J. Biol. Chem., 271, 7544–7550. [DOI] [PubMed] [Google Scholar]
- Clemetson J.M., Polgar,J., Magnenat,E., Wells,T.N.C. and Clemetson,K.J. (1999) The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J. Biol. Chem., 274, 29019–29024. [DOI] [PubMed] [Google Scholar]
- Dachary-Prigent J., Freyssinet,J.-M., Pasquet,J.-M., Carron,J.-C. and Nurden,A.T. (1993) Annexin V as a probe of aminophospholipid exposure and platelet membrane vesiculation: a flow cytometry study showing a role for free sulphydryl groups. Blood, 81, 2554–2565. [PubMed] [Google Scholar]
- Damen J.E., Liu,L., Ware,M.D., Ermolaeva,M., Majerus,P.W. and Krystal,G. (1998) Multiple forms of the SH2-containing inositol phosphatase, SHIP, are generated by C-terminal truncation. Blood, 92, 1199–1205. [PubMed] [Google Scholar]
- Ezumi Y., Shindoh,K., Tsuji,M. and Takayama,H. (1998) Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor γ chain complex on human platelets. J. Exp. Med., 188, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M., Logan,S.K., Lehto,V.P., Baccante,G., Lemmon,M.A. and Schlessinger,J. (1998) Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J., 17, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger A.C. et al. (1998) Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J., 17, 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke T.F., Kaplan,D.R., Cantley,L.C. and Toker,A. (1997) Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science, 275, 665–668. [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Meyers,R.E. and Cantley,L.C. (1998) Phosphoinositide kinases. Annu. Rev. Biochem., 67, 481–507. [DOI] [PubMed] [Google Scholar]
- Fruman D., Snapper,S., Yballe,C.M., Davidson,L., Yu,J., Alt,F. and Cantley,L. (1999) Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science, 283, 393–397. [DOI] [PubMed] [Google Scholar]
- Gibbins J.M., Okuma,M., Farndale,R., Barnes,M. and Watson,S.P. (1997) Glycoprotein VI is the collagen receptor in platelets which underlie tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett., 413, 255–259. [DOI] [PubMed] [Google Scholar]
- Giurato S., Payrastre,B., Drayer,L.A., Plantavid,M., Woscholski,R., Parker,P., Erneux,C. and Chap,H. (1997) Tyrosine phosphorylation and relocation of SHIP are integrin-mediated in thrombin-stimulated human blood platelets. J. Biol. Chem., 272, 26857–26863. [DOI] [PubMed] [Google Scholar]
- Gratacap M.-P., Payrastre,B., Viala,C., Mauco,G., Plantavid,M. and Chap,H. (1998) Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-γ2 is an early key event in FcγRIIA-mediated activation of human platelets. J. Biol. Chem., 273, 24314–24321. [DOI] [PubMed] [Google Scholar]
- Gross B., Lee,J.R., Clements,J.L., Turner,M., Tybulewicz,V.L.J., Findell,P.R., Koretzky,G.A. and Watson,S.P. (1999a) Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor GPVI in platelets. J. Biol. Chem., 274, 5963–5971. [DOI] [PubMed] [Google Scholar]
- Gross B.S., Melford,S.K. and Watson,S.P. (1999b) Evidence that phospholipase C-γ2 interacts with SLP-76, Syk, LAT, Lyn and the FcR γ-chain after stimulation of the collagen receptor glycoprotein VI in human platelets. Eur. J. Biochem., 263, 612–623. [DOI] [PubMed] [Google Scholar]
- Helgason C.D. et al. (1998) Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology and a shortened life span. Genes Dev., 12, 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Kucera,G.L. and Rittenhouse,S.E. (1991) Elevated cytosolic Ca2+ activates phospholipase D in human platelets. J. Biol. Chem., 266, 1652–1655. [PubMed] [Google Scholar]
- Huber M., Helgason,C.D., Damen,J.E., Liu,L., Humphries,R.K. and Krystal,G. (1998a) The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl Acad. Sci. USA, 95, 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M., Helgason,C.D., Scheid,M.P., Duronio,V., Humphries,R.K. and Krystal,G. (1998b) Targeted disruption of SHIP leads to steel factor-induced degranulation of mast cells. EMBO J., 17, 7311–7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrel B., Wierwille,S., Clemetson,K.J., Anders,O., Steiner,M., Knight,C.G., Farndale,R.W., Okuma,M. and Barnes,M.J. (1998) Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa and von Willebrand factor do not. Blood, 91, 491–499. [PubMed] [Google Scholar]
- Laffargue M. et al. (1998) Phosphoinositide 3-kinase and integrin signalling are involved in activation of Bruton’s tyrosine kinase in thrombin-stimulated platelets. FEBS Lett., 443, 66–70. [DOI] [PubMed] [Google Scholar]
- Li Z., Wahl,M.I., Eguinoa,A., Stephens,L.R., Hawkins,P.T. and Witte,O.N. (1997) Phosphatidylinositol 3-kinase-γ activates Bruton’s tyrosine kinase in concert with Src family kinases. Proc. Natl Acad. Sci. USA, 94, 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P.-J., Hsu,A.-L., Wang,D.-S. and Chen,C.-S. (1998) Phosphatidylinositol 3,4,5-trisphosphate triggers platelet aggregation by activating Ca2+ influx. Biochemistry, 37, 9776–9783. [DOI] [PubMed] [Google Scholar]
- Mahajan S., Fargnoli,J., Burkhardt,A.L., Kut,S.A., Saouaf,S.J. and Bolen,J.B. (1995) Src family protein tyrosine kinases induce autoactivation of Bruton’s tyrosine kinase. Mol. Cell. Biol., 15, 5304–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melford S.K., Turner,M., Briddon,S.J., Tybulewicz,V.L.J. and Watson,S.P. (1997) Syk and Fyn are required by mouse megakaryocytes for the rise in intracellular calcium induced by a collagen-related peptide. J. Biol. Chem., 272, 27539–27542. [DOI] [PubMed] [Google Scholar]
- Morton L.F., Hargreaves,P.G., Farndale,R.W., Young,R.D. and Barnes,M.J. (1995) Integrin α2 β1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for α2 β1-independent platelet reactivity. Biochem. J., 306, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet J.-M., Bobe,R., Gross,B., Gratacap,M.-P., Tomlinson,M.G., Payrastre,P. and Watson,S.P. (1999a) A collagen related peptide regulates phospholipase Cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem. J., 342, 171–177. [PMC free article] [PubMed] [Google Scholar]
- Pasquet J.-M. et al. (1999b) Lat is required for tyrosine phosphorylation of phospholipase Cγ2 and platelet activation by the collagen receptor GPVI. Mol. Cell. Biol., 19, 8326–8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesesse X., Moreau,C., Drayer,A.L., Woscholski,R., Parker,P. and Erneux,C. (1998) The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett., 437, 301–303. [DOI] [PubMed] [Google Scholar]
- Quek L.S., Bolen,J. and Watson,S.P. (1998) Regulation of Bruton’s tyrosine kinase and phospholipase Cγ2 in collagen-stimulated platelets. Curr. Biol., 8, 1137–1140. [DOI] [PubMed] [Google Scholar]
- Rameh L.E. et al. (1997) A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J. Biol. Chem., 272, 22059–22066. [DOI] [PubMed] [Google Scholar]
- Rawlings D.J., Scharenberg,A.M., Park,H., Wahl,M., Lin,S., Kato,R.M., Fluckiger,A.-C., Witte,O.N. and Kinet,J.-P. (1996) Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science, 271, 822–825. [DOI] [PubMed] [Google Scholar]
- Salim K. et al. (1996) Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dymanic and Bruton’s tyrosine kinase. EMBO J., 15, 6241–6250. [PMC free article] [PubMed] [Google Scholar]
- Scharenberg A.M. and Kinet,J. (1998) Ptdlns-3,4,5-P3: A regulatory nexus between tyrosine kinases and sustained calcium signals. Cell, 94, 5–8. [DOI] [PubMed] [Google Scholar]
- Scharenberg A.M. et al. (1998) Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5P3)/Tec kinase dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J., 17, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Terauchi,Y., Fujiwara,M., Aizawa,S., Yazaki,Y., Kadowaki,T. and Koyasu,S. (1999) Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science, 283, 390–392. [DOI] [PubMed] [Google Scholar]
- Takata M. and Kurosaki,T. (1996) A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med., 184, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M., Ezumi,Y., Arai,M. and Takayama,H. (1997) A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J. Biol. Chem., 272, 23528–23531. [DOI] [PubMed] [Google Scholar]
- Tsukada S. et al. (1993) Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobinemia. Cell, 72, 279–290. [DOI] [PubMed] [Google Scholar]
- Turner M., Mee,P.J., Costello,P.S., Williams,O., Price,A.A., Duddy,L.P., Furlong,M.T., Geahlen,R.L. and Tybulewicz,V.L.J. (1995) Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature, 378, 298–302. [DOI] [PubMed] [Google Scholar]
- Watson S.P. and Gibbins,J. (1998) Collagen receptor signalling in platelets: extending the role of the ITAM. Immunol. Today, 19, 260–265. [DOI] [PubMed] [Google Scholar]
- Zhang J., Banfic,H., Straforini,F., Tosi,L., Volinia,S. and Rittenhouse,S.E. (1998) A type ii phosphoinositide 3-kinase is stimulated via activated integrin in platelets. J. Biol. Chem., 273, 14081–14084. [DOI] [PubMed] [Google Scholar]