Abstract
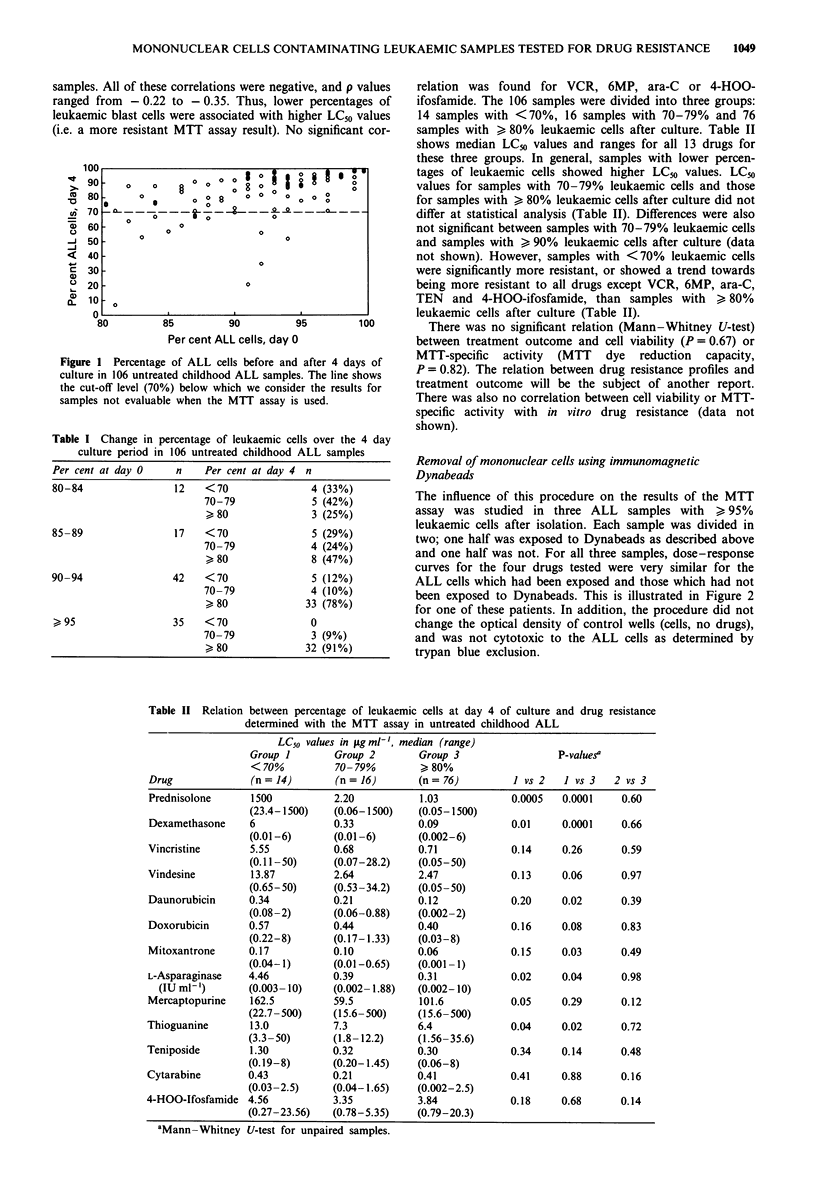
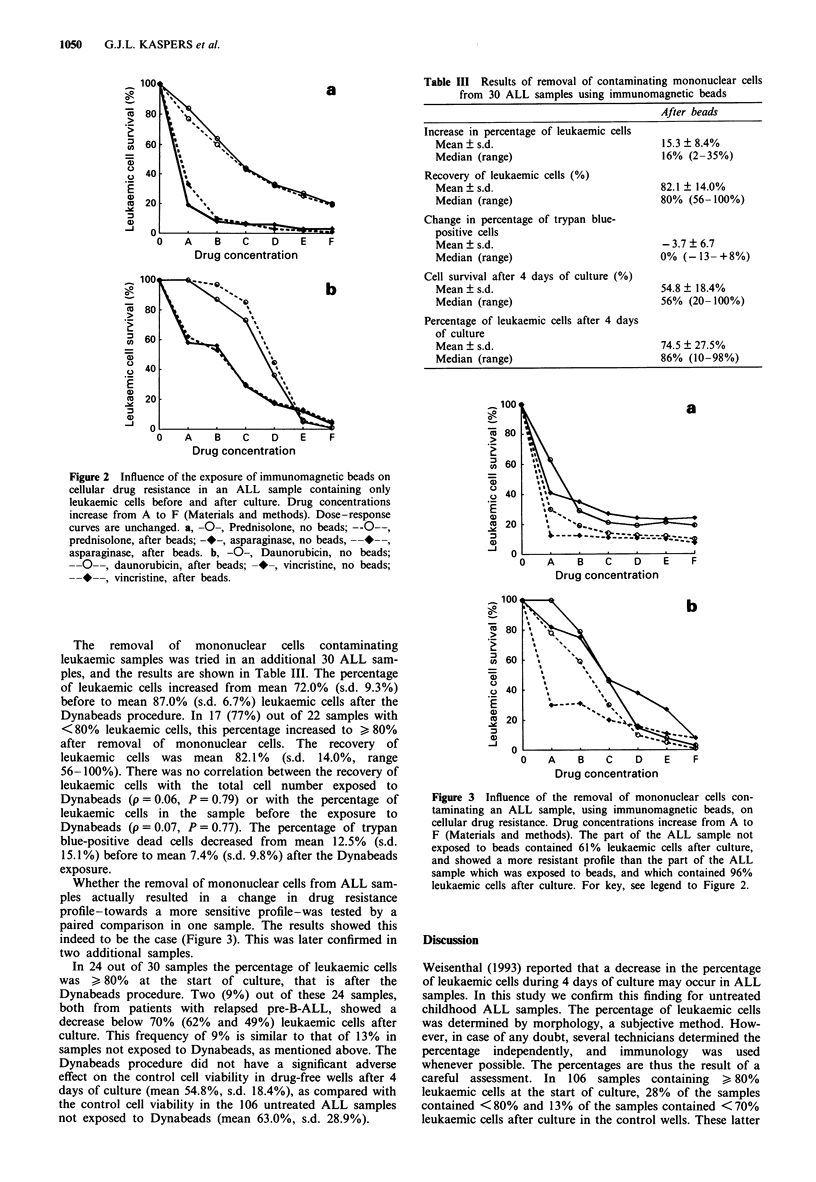
The methyl-thiazol-tetrazolium (MTT) assay is a drug resistance assay which cannot discriminate between malignant and non-malignant cells. We previously reported that samples with > or = 80% leukaemic cells at the start of culture give similar results in the MTT assay and the differential staining cytotoxicity assay, in which a discrimination between malignant and non-malignant cells can be made. However, the percentage of leukaemic cells may change during culture, which might affect the results of the MTT assay. We studied 106 untreated childhood acute lymphoblastic leukemia (ALL) samples with > or = 80% leukaemic cells at the start of culture. This percentage decreased below 80% in 28%, and below 70% in 13%, of the samples after 4 days of culture. A decrease below 70% occurred more often in case of 80-89% leukaemic cells (9/29) than in case of > or = 90% leukaemic cells at the start of culture (5/77, P = 0.0009). Samples with < 70% leukaemic cells after culture were significantly more resistant to 6 out of 13 drugs, and showed a trend towards being more resistant to two more drugs, than samples with > or = 80% leukaemic cells. No such differences were seen between samples with 70-79% and samples with > or = 80% leukaemic cells after culture. We next studied in another 30 ALL samples whether contaminating mononuclear cells could be removed by using immunoamagnetic beads. Using a beads to target cell ratio of 10:1, the percentage of leukaemic cells increased from mean 72% (s.d. 9.3%) to mean 87% (s.d. 6.7%), with an absolute increase of 2-35%. The recovery of leukaemic cells was mean 82.1% (range 56-100%, s.d. 14.0%). The procedure itself did not influence the results of the MTT assay in three samples containing only leukaemic cells. We conclude that it is important to determine the percentage of leukaemic cells at the start and at the end of the MTT assay and similar drug resistance assays. Contaminating mononuclear cells can be successfully removed from ALL samples using immunomagnetic beads. This approach may increase the number of leukaemic samples which can be evaluated for cellular drug resistance with the MTT assay or a similar cell culture drug resistance assay.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK M. M., SPEER F. D. Effects of cancer chemotherapeutic agents on dehydrogenase activity of human cancer tissue in vitro. Am J Clin Pathol. 1953 Mar;23(3):218–227. doi: 10.1093/ajcp/23.3.218. [DOI] [PubMed] [Google Scholar]
- Campling B. G., Pym J., Galbraith P. R., Cole S. P. Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leuk Res. 1988;12(10):823–831. doi: 10.1016/0145-2126(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Gruhn B., Häfer R., Müller A., Andrä W., Danan H., Zintl F. Model experiments for immunomagnetic elimination of leukemic cells from human bone marrow. Presentation of a novel magnetic separation system. Immunobiology. 1991 Nov;183(5):374–385. doi: 10.1016/S0171-2985(11)80522-X. [DOI] [PubMed] [Google Scholar]
- Hongo T., Fujii Y., Igarashi Y. An in vitro chemosensitivity test for the screening of anti-cancer drugs in childhood leukemia. Cancer. 1990 Mar 15;65(6):1263–1272. doi: 10.1002/1097-0142(19900315)65:6<1263::aid-cncr2820650602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hongo T., Fujii Y., Mizuno Y., Haraguchi S., Yoshida T. O. [Anticancer drug sensitivity test using the short-term microplate culture and MTT dye reduction assay]. Gan To Kagaku Ryoho. 1987 Feb;14(2):472–478. [PubMed] [Google Scholar]
- Hwang W. S., Chen L. M., Huang S. H., Wang C. C., Tseng M. T. Prediction of chemotherapy response in human leukemia using in vitro chemosensitivity test. Leuk Res. 1993 Aug;17(8):685–688. doi: 10.1016/0145-2126(93)90074-u. [DOI] [PubMed] [Google Scholar]
- Kaspers G. J., Kardos G., Pieters R., Van Zantwijk C. H., Klumper E., Hählen K., de Waal F. C., van Wering E. R., Veerman A. J. Different cellular drug resistance profiles in childhood lymphoblastic and non-lymphoblastic leukemia: a preliminary report. Leukemia. 1994 Jul;8(7):1224–1229. [PubMed] [Google Scholar]
- Kaspers G. J., Pieters R., Van Zantwijk C. H., De Laat P. A., De Waal F. C., Van Wering E. R., Veerman A. J. In vitro drug sensitivity of normal peripheral blood lymphocytes and childhood leukaemic cells from bone marrow and peripheral blood. Br J Cancer. 1991 Sep;64(3):469–474. doi: 10.1038/bjc.1991.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D. L., Duke M., Goh T. S. Chemosensitivity testing of fresh human leukemia cells using both a dye exclusion assay and a tetrazolium dye (MTT) assay. Leuk Res. 1990;14(5):459–466. doi: 10.1016/0145-2126(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Larsson R., Kristensen J., Sandberg C., Nygren P. Laboratory determination of chemotherapeutic drug resistance in tumor cells from patients with leukemia, using a fluorometric microculture cytotoxicity assay (FMCA). Int J Cancer. 1992 Jan 21;50(2):177–185. doi: 10.1002/ijc.2910500204. [DOI] [PubMed] [Google Scholar]
- Linehan M., Miller E., Anglard P., Merino M., Zbar B. Improved detection of allele loss in renal cell carcinomas after removal of leukocytes by immunologic selection. J Natl Cancer Inst. 1989 Feb 15;81(4):287–290. doi: 10.1093/jnci/81.4.287. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Leyva A., Veerman A. J. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Lett. 1988 Aug 30;41(3):323–332. doi: 10.1016/0304-3835(88)90294-7. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Leyva A., Veerman A. J. Comparison of the rapid automated MTT-assay with a dye exclusion assay for chemosensitivity testing in childhood leukaemia. Br J Cancer. 1989 Feb;59(2):217–220. doi: 10.1038/bjc.1989.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Loonen A. H., Hählen K., van der Does-van den Berg A., van Wering E. R., Veerman A. J. Relation of cellular drug resistance to long-term clinical outcome in childhood acute lymphoblastic leukaemia. Lancet. 1991 Aug 17;338(8764):399–403. doi: 10.1016/0140-6736(91)91029-t. [DOI] [PubMed] [Google Scholar]
- Pieters R., Loonen A. H., Huismans D. R., Broekema G. J., Dirven M. W., Heyenbrok M. W., Hählen K., Veerman A. J. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions. Blood. 1990 Dec 1;76(11):2327–2336. [PubMed] [Google Scholar]
- Santini V., Bernabei P. A., Silvestro L., Dal Pozzo O., Bezzini R., Viano I., Gattei V., Saccardi R., Ferrini P. R. In vitro chemosensitivity testing of leukemic cells: prediction of response to chemotherapy in patients with acute non-lymphocytic leukemia. Hematol Oncol. 1989 Jul-Aug;7(4):287–293. doi: 10.1002/hon.2900070405. [DOI] [PubMed] [Google Scholar]
- Sargent J. M., Taylor C. G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989 Aug;60(2):206–210. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki C., Wisniewski D., Scheinberg D. A., Atzpodien J., Strife A., Gulati S., Fried J., Wisniewolski R., Wang C. Y., Clarkson B. D. Elimination of myeloma cells from bone marrow by using monoclonal antibodies and magnetic immunobeads. Blood. 1988 Oct;72(4):1248–1254. [PubMed] [Google Scholar]
- Skjønsberg C., Erikstein B. K., Smeland E. B., Lie S. O., Funderud S., Beiske K., Blomhoff H. K. Interleukin-7 differentiates a subgroup of acute lymphoblastic leukemias. Blood. 1991 Jun 1;77(11):2445–2450. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Rees J. K. Chemosensitivity testing of fresh leukaemia cells using the MTT colorimetric assay. Br J Haematol. 1989 Jan;71(1):19–24. doi: 10.1111/j.1365-2141.1989.tb06268.x. [DOI] [PubMed] [Google Scholar]
- Vartdal F., Kvalheim G., Lea T. E., Bosnes V., Gaudernack G., Ugelstad J., Albrechtsen D. Depletion of T lymphocytes from human bone marrow. Use of magnetic monosized polymer microspheres coated with T-lymphocyte-specific monoclonal antibodies. Transplantation. 1987 Mar;43(3):366–371. [PubMed] [Google Scholar]
- Veerman A. J., Pieters R. Drug sensitivity assays in leukaemia and lymphoma. Br J Haematol. 1990 Apr;74(4):381–384. doi: 10.1111/j.1365-2141.1990.tb06323.x. [DOI] [PubMed] [Google Scholar]
- Weisenthal L. M., Kern D. H. Prediction of drug resistance in cancer chemotherapy: the Kern and DiSC assays. Oncology (Williston Park) 1991 Sep;5(9):93-103; disc. 104, 111-4, 117-8. [PubMed] [Google Scholar]
- Weisenthal L. M., Lippman M. E. Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat Rep. 1985 Jun;69(6):615–632. [PubMed] [Google Scholar]


