Abstract
Lipids have been implicated in signal transduction and in several stages of membrane trafficking, but these two functions have not been functionally linked. In yeast, sphingoid base synthesis is required for the internalization step of endocytosis and organization of the actin cytoskeleton. We show that inactivation of a protein phosphatase 2A (PP2A) or overexpression of one of two kinases, Yck2p or Pkc1p, can specifically suppress the sphingoid base synthesis requirement for endocytosis. The two kinases have an overlapping function because only a mutant with impaired function of both kinases is defective in endocytosis. An ultimate target of sphingoid base synthesis may be the actin cytoskeleton, because overexpression of the kinases and inactivation of PP2A substantially corrected the actin defect due to the absence of sphingoid base. These results suggest that sphingoid base controls protein phosphorylation, perhaps by activating a signal transduction pathway that is required for endocytosis and proper actin cytoskeleton organization in yeast.
Keywords: casein kinase I/endocytosis/protein kinase C/protein phosphatase 2A/sphingoid base
Introduction
Endocytosis is the process whereby eukaryotic cells internalize both plasma membrane and extracellular material. It is a commonly used pathway for recycling of components used in the secretory pathway, for the uptake of micronutrients and for the down-regulation of cell surface receptors. Certain viruses and microbes also exploit it to gain access to internal cellular compartments. Genetic studies of endocytosis using the yeast Saccharomyces cerevisiae have identified several components required for this pathway (Riezman et al., 1996; Geli and Riezman, 1998). Recently, not only proteins but also lipids, like sterols, were shown to be required for the internalization step of the endocytic pathway in yeast (Munn et al., 1999). Sterols also play a role in endocytosis in animal cells (Anderson, 1998; Kobayashi et al., 1998; Rodal et al., 1999; Subtil et al., 1999). Moreover, phosphatidic acid, diacylglycerol (DAG), ceramides and phosphoinositides have been implicated in several stages of membrane trafficking other than endocytosis in yeast (Bankaitis et al., 1990; Schu et al., 1993; Horvath et al., 1994; Kearns et al., 1997).
Sphingolipids and sphingoid bases and their phosphorylated derivatives, dihydrosphingosine-1-P (DHS-1P) and phytosphingosine-1-P (PHS-1P), are thought to be signaling molecules for regulating a variety of mammalian cellular processes including cell growth, stress, differentiation and apoptosis (Hannun, 1994, 1996; Cuvillier et al., 1996; Jayadev and Hannun, 1996; Kolesnick and Hannun, 1999). In mammalian cells, studies revealed that sphingosine induces in vitro phosphorylation of endogenous proteins through the activation of protein kinases (Pushkareva et al., 1992), and two unidentified sphingosine-activated protein kinases could be distinguished by their substrate specificity and their sphingosine requirement (Pushkareva et al., 1993). Sphingosine was also shown to have the ability to inhibit some kinases like protein kinase C (Hannun et al., 1986) and to activate some others, including casein kinase II (McDonald et al., 1991), the atypical protein kinase C isoform ζ (Muller et al., 1995), p21 activated kinase-1 (PAK1) (Bokoch et al., 1998) and 3-phosphoinositide-dependent kinase-1 (PDK1) (King et al., 2000). Thus, sphingoid bases appear to be bifunctional molecules with the ability to regulate protein phosphorylation.
In S.cerevisiae, sphingoid bases have been implicated in the heat stress response in yeast because they accumulate during heat stress (Jenkins et al., 1997; Skrzypek et al., 1999). Treatment of yeast cells with dihydrosphingosine (DHS), an intermediate in ceramide synthesis, leads to an accumulation of trehalose, a disaccharide that is required for full protection against heat stress. Moreover, DHS treatment induces transcription of a reporter gene containing the TPS2 promoter or STRE (stress response element) sequences. The TPS2 gene encodes a subunit of the trehalose synthase complex, suggesting that DHS acts as a signaling molecule to regulate trehalose accumulation during the heat stress response (Dickson et al., 1997). Recent reports show that heat shock induces an increase in the concentration of two phosphorylated sphingoid bases DHS-1P and PHS-1P (Skrzypek et al., 1999). In addition, several mutants accumulating these compounds have an increased survival at elevated temperature, suggesting that DHS-1P and PHS-1P may act as signals for resistance to heat stress (Mandala et al., 1998; Mao et al., 1999; Skrzypek et al., 1999).
Using the S.cerevisiae lcb1-100 mutant, a requirement for sphingoid base synthesis for the internalization step of endocytosis and for proper actin cytoskeleton organization was revealed (Zanolari et al., 2000). The LCB1 gene encodes a subunit of serine palmitoyltransferase that catalyzes the first step in sphingolipid synthesis: the condensation of serine and palmitoyl-CoA to yield the 3-ketosphinganine (Nagiec et al., 1994). Here we show that inactivation of a protein phosphatase, PP2A, or overexpression of one of two protein kinases, Yck2p or Pkc1p, can abrogate this novel lipid requirement for endocytosis and restore a proper organization of the actin cytoskeleton. The two kinases have an overlapping function in endocytosis because only a mutant with impaired function of both kinases is defective in the internalization step of endocytosis. These results imply that the function of sphingoid base synthesis in endocytosis is to control protein phosphorylation. The ultimate target of the sphingoid base requirement may be the endocytic machinery and/or the actin cytoskeleton.
Results
Mutations in subunits of PP2A do not lead to a defect in endocytosis
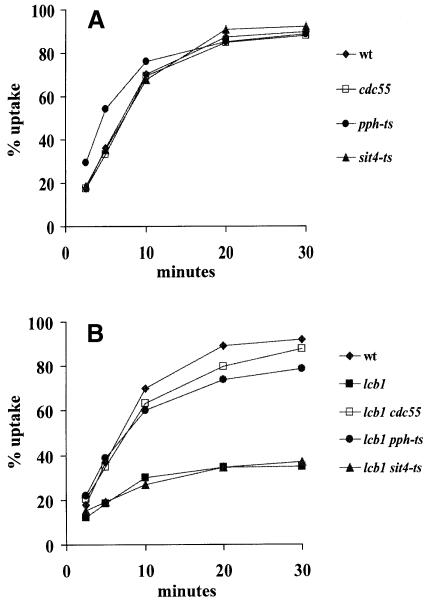
Sphingolipids have been proposed to activate protein phosphatases (Dobrowsky et al., 1993; Wolff et al., 1994). The best candidate for a ceramide-activated protein phosphatase (CAPP) in yeast is PP2A (Fishbein et al., 1993; Nickels and Broach, 1996), which has two regulatory subunits, Cdc55p and Tpd3p (Healy et al., 1991; van Zyl et al., 1992), and a catalytic subunit. The identity of the catalytic subunit has been postulated to be Sit4p for the yeast CAPP (Nickels and Broach, 1996), but three other functionally overlapping genes, PPH21, PPH22 and PPH3, encode the majority of the PP2A catalytic activity in yeast (Sneddon et al., 1990; Ronne et al., 1991). To determine whether PP2A plays a role in endocytosis, we constructed deletion strains for the non-essential regulatory subunit Cdc55p and temperature-sensitive strains for the essential catalytic subunits in our strain background. The strain pph-ts contains deletions of the catalytic subunits (pph21Δ pph22Δ pph3Δ) and is kept alive by a plasmid-borne temperature-sensitive pph21-102 mutant allele. The strain sit4-ts contains a deletion of the catalytic subunit, sit4Δ, and harbors a temperature-sensitive sit4-102 allele on a plasmid. In yeast, the internalization step of receptor-mediated endocytosis can be followed by using the α-factor pheromone that is bound and internalized by its G-protein coupled receptor, Ste2p (Riezman, 1998). α-factor uptake at 37°C was determined for cdc55Δ, pph-ts and sit4-ts strains and compared with wild-type cells (Figure 1A). All strains showed wild-type internalization kinetics, indicating that the internalization step of endocytosis is not affected by impaired function in a catalytic or a regulatory subunit of the PP2A.
Fig. 1. Loss of PP2A function suppresses the lcb1-100 mutation for endocytosis. (A) Wild-type RH1800 (wt) and single mutant strains RH3745 (cdc55), RH4137 (pph-ts) and RH4183 (sit4-ts) were assayed for α-factor internalization at 37°C. All mutants showed almost wild-type internalization kinetics. (B) The RH3809 strain (lcb1-100) and double mutant strains, RH3807 (lcb1 cdc55), RH4191 (lcb1 pph-ts) and RH4195 (lcb1 sit4-ts) were assayed for α-factor internalization at 37°C.
Mutations in PP2A subunits restore endocytosis in the lcb1-100 mutant
The yeast lcb1-100 mutant has a temperature-sensitive defect in the internalization step of endocytosis, witnessed by a defect in accumulation of the fluid-phase marker, lucifer yellow carbohydrazide (LY), in the vacuole (Figure 3) and a defect in internalization of the yeast α-factor pheromone (Figure 1B) (Munn and Riezman, 1994). To check whether inactivation of PP2A can affect the sphingoid base synthesis requirement for endocytosis, we created lcb1 cdc55Δ, lcb1 pph-ts and lcb1 sit4-ts mutant strains. We assayed these strains for internalization of α-factor at 37°C (Figure 1B). Interestingly, the lcb1 cdc55Δ and lcb1 pph-ts mutants internalized α-factor to a similar extent to the wild-type strain but the lcb1 sit4-ts mutant was still clearly defective in α-factor uptake. These results indicate that inactivation of the CDC55 or the PPH catalytic subunits of PP2A restores receptor-mediated endocytosis in the lcb1-100 mutant.
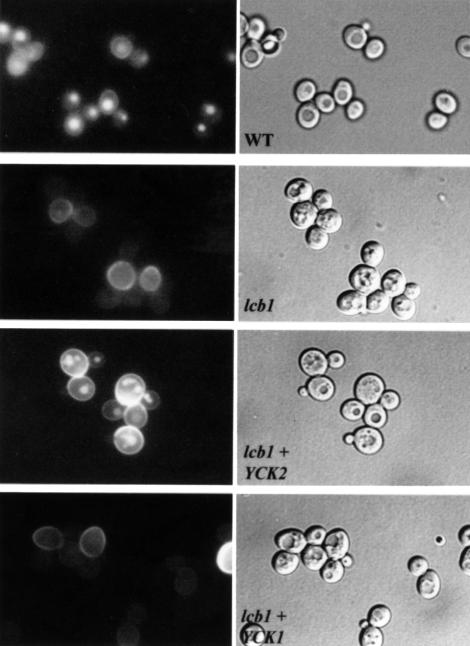
Fig. 3. YCK2 overexpression suppresses the fluid-phase endocytic defect of the lcb1-100 mutant. Wild-type cells (WT, RH448) and lcb1-100 (lcb1, RH3802) cells carrying either a YCK2 or a YCK1 high copy number plasmid (lcb1+YCK2 or lcb1+YCK1) were assayed for LY accumulation in the vacuole at 37°C. The same field of cells viewed by fluorescence (left panels) and by Nomarski optics (right panels) is shown. Note that lcb1-100 cells have fragmented vacuoles when compared with wild-type cells (right panels).
These results were further supported by fluid-phase endocytosis assays. The lcb1 cdc55Δ and lcb1 pph-ts mutants accumulated LY in the vacuole to similar levels to wild-type cells (data not shown). These results show that loss of CDC55 and PPH21/22/3 function completely suppresses the lcb1-100 mutation for endocytosis. Thus, the loss of PP2A activity abrogated the sphingoid base synthesis requirement for endocytosis, suggesting that the major role of sphingoid base is control of the protein phosphorylation status.
Overexpression of one of two kinases restores endocytosis in the lcb1-100 mutant
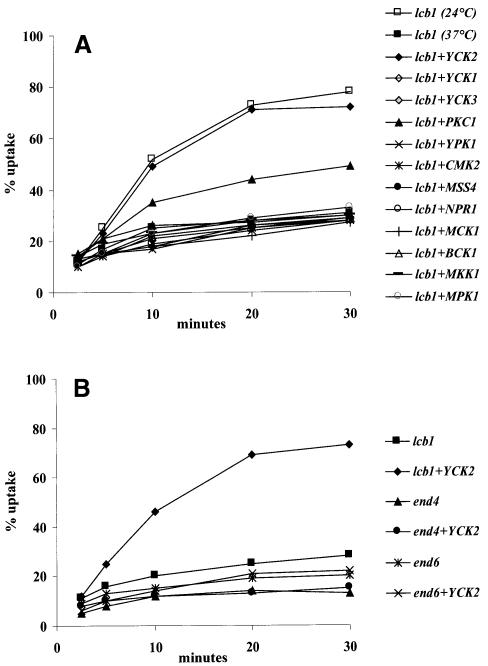
To identify a kinase that could control protein phosphorylation for endocytosis, we searched for a protein kinase whose overexpression would suppress the endocytic defect of the lcb1-100 mutant. The lcb1-100 strain was transformed with high copy (2µ) plasmids bearing genes encoding protein kinases (Table I). The yeast protein kinases can be subdivided into distinct families based on structural similarity in their catalytic domains (Hunter and Plowman, 1997). The kinases tested in this study belong to several families of protein kinases (Table I). None of the protein kinases tested was able to suppress the temperature-sensitive growth phenotype displayed by the lcb1-100 mutant (data not shown). The strains overexpressing different protein kinases were tested for internalization of [35S]α-factor at 37°C. As a positive control, we measured α-factor internalization by lcb1-100 cells at 24°C, because this mutant shows only a partial defect in endocytosis at 24°C (Figure 2A). Most of the protein kinases tested did not suppress the lcb1-100 defect in endocytosis. However, high copy vectors with two protein kinases from different families, Yck2p and Pkc1p, did. To characterize further the suppressor effects of these two kinases, more assays were performed.
Table I. Plasmids used in this study.
| Plasmid | Yeast ori | Insert | Function | Group | Reference |
|---|---|---|---|---|---|
| pLJ721 | 2µ | YCK1 | casein kinase I isoform | CK1 | Robinson et al. (1993) |
| pL2.3 | 2µ | YCK2 | casein kinase I isoform | CK1 | Robinson et al. (1992) |
| YEp24-YCK3 | 2µ | YCK3 | casein kinase I isoform | CK1 | L.C.Robinson |
| pSH24 | 2µ | PKC1 | protein kinase C | AGC | Helliwell et al. (1998b) |
| YCp-PKC1(R398P) | CEN | PKC1(R398P) | protein kinase C dominant activating mutant | AGC | Nonaka et al. (1995) |
| pSMA10 | 2µ | BCK1 | member of the Pkc1p MAP kinase module | STE11/STE20 | Irie et al. (1991) |
| YEp352-MKK1 | 2µ | MKK1 | MEK, MAP kinase kinase | STE7/MEK | D.E.Levin |
| YEp352-MPK1 | 2µ | MPK1 | MAP kinase | CMGC | Kamada et al. (1995) |
| YEp352-YPK1 | 2µ | YPK1 | protein kinase with similarity to Pkc1p | AGC | Chen et al. (1993) |
| pSH22 | 2µ | MSS4 | phosphatidylinositol-4-phosphate 5-kinase | PI-kinase | Helliwell et al. (1998a) |
| pSEY18-CMK2 | 2µ | CMK2 | calcium–calmodulin-dependent kinase type II | CaMK | T.Beck |
| pJK20 | 2µ | NPR1 | Ser/Thr kinase, nitrogen permease reactivator | NPR | Schmidt et al. (1998) |
| pSEY18-MCK1 | 2µ | MCK1 | meiosis and centromere regulatory kinase | CMGC | T.Beck |
The classification of protein kinases in different groups is based on Hunter and Plowman (1997).
Fig. 2. Overexpression of YCK2 and PKC1 specifically suppresses the lcb1-100 endocytic defect. (A) Strain RH3802 (lcb1) was transformed with high copy plasmids carrying the indicated genes (Table I) and the corresponding transformants were assayed for α-factor internalization at 37°C and compared with lcb1-100 cells at 24 and 37°C. (B) The lcb1-100, end4-1 (sla2-41) and end6-1 (rvs161) temperature-sensitive mutants (RH3802, RH1597 and RH2082) were transformed by a high copy number plasmid bearing YCK2 and assayed for α-factor uptake at 37°C.
To check whether suppression by Yck2p was specific, two other kinases, Yck1p and Yck3p, which also belong to the casein kinase I family (Robinson et al., 1992; Wang et al., 1996), were tested. Neither of these two kinases was able to restore α-factor internalization in the lcb1-100 mutant (Figure 2A). The ability of lcb1-100 cells overexpressing YCK2 or YCK1 to carry out fluid-phase endocytosis at 37°C was also tested and compared with wild-type and lcb1-100 cells (Figure 3). The lcb1-100 mutant showed a few small vacuoles when viewed by Nomarski optics and was defective for accumulation of LY in the vacuole. Consistent with the α-factor uptake results, overexpression of YCK2 allowed the lcb1-100 mutant to accumulate LY in the vacuole, while overexpression of YCK1 did not (Figure 3). To determine whether other endocytosis mutants could also be suppressed by YCK2 overexpression, the end4-1 (sla2-41) and end6-1 (rvs161) strains were transformed by the 2µ plasmid bearing the YCK2 gene and assayed for α-factor uptake at 37°C (Figure 2B). These two mutants are defective for α-factor internalization at 37°C (Raths et al., 1993; Munn et al., 1995). The high copy expression of YCK2 did not restore endocytosis in these strains (Figure 2B). We conclude that Yck2p overexpression specifically suppresses the lcb1-100 endocytic defect.
The other protein kinase that restored endocytosis in the lcb1-100 mutant was protein kinase C. Overexpression of PKC1 permitted lcb1-100 cells to internalize 50% of the α-factor by 30 min at 37°C. The lcb1-100 mutant, with or without multicopy vectors bearing other kinases, was defective for α-factor uptake at 37°C (25–30% uptake after 30 min; Figure 2A). We also overproduced protein kinase C activity by transformation with a low copy number plasmid bearing a dominant, activated allele of PKC1 (PKC1-R398P) (Nonaka et al., 1995) and measured α-factor uptake at 37°C. PKC1-R398P expression was able to restore α-factor uptake by lcb1-100 cells to similar levels to overexpression of PKC1 (data not shown). These results suggest that the lcb1-100 mutant could be impaired in activation of Pkc1p and furthermore in the characterized PKC1-mediated signaling pathway.
Genetic studies have shown that one of the downstream signaling pathways of Pkc1p is the mitogen-activated protein (MAP) kinase cascade (Herskowitz, 1995). This MAP kinase signaling pathway is composed of four downstream effectors, Bck1p, Mkk1p/Mkk2p and Mpk1p (Lee and Levin, 1992; Irie et al., 1993; Lee et al., 1993), which are homologs of MAP kinase kinase kinase, MAP kinase kinase and MAP kinase in mammalian cells, respectively. The lcb1-100 mutant was transformed with high copy number plasmids bearing BCK1, MKK1 or MPK1 genes, and α-factor uptake was assayed at 37°C (Figure 2A). None of these three kinases was able to suppress the endocytic defect of the lcb1-100 cells, indicating that the suppressor effect of the Pkc1p kinase is not mediated through the known MAP kinase pathway.
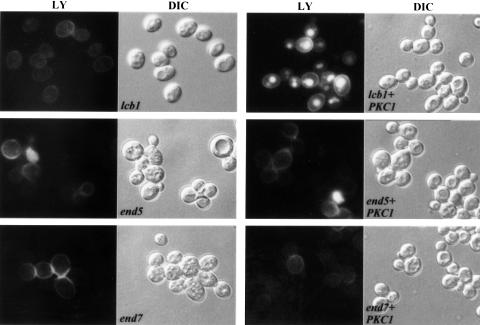
To assay fluid-phase endocytosis, lcb1-100 cells bearing the PKC1 2µ plasmid were incubated with LY for 1 h at 37°C (Figure 4). Overexpression of PKC1 restored LY accumulation by the lcb1-100 mutant very efficiently. To test whether the suppression is specific for the sphingoid base synthesis requirement, LY accumulation was measured in other temperature-sensitive endocytosis deficient mutants, end4-1 (sla2-41) (Raths et al., 1993), end6-1 (rvs161), end5-1 (vrp1) and end7-1 (act1) (Munn et al., 1995) transformed with the PKC1 plasmid (data not shown and Figure 4). The endocytic defect of these mutants was not suppressed by PKC1. Taken together, the above results show that overexpression of two kinases, Yck2p and Pkc1p, can suppress both receptor-mediated and fluid-phase endocytosis defects of the lcb1-100 mutant. This suppressor effect is specific for the sphingoid base synthesis requirement, because other endocytic mutants were not suppressed by high copy expression of these kinases. Therefore, sphingoid base synthesis may activate a protein phosphorylation pathway that is required for the internalization step of endocytosis.
Fig. 4. Overexpression of PKC1 specifically suppresses the LY accumulation defect of the lcb1-100 mutant. The lcb1-100, end5-1 (vrp1) and end7-1 (act1) temperature-sensitive mutants (RH3802, RH2077 and RH2069), which are defective in fluid-phase endocytosis (left panels) (Raths et al., 1993; Munn et al., 1995), were transformed by a high copy number plasmid containing PKC1 (right panels), assayed for LY accumulation at 37°C and observed by fluorescence (LY) and Nomarski (DIC) microscopy.
Genetic interaction between lcb1-100 and yck-ts
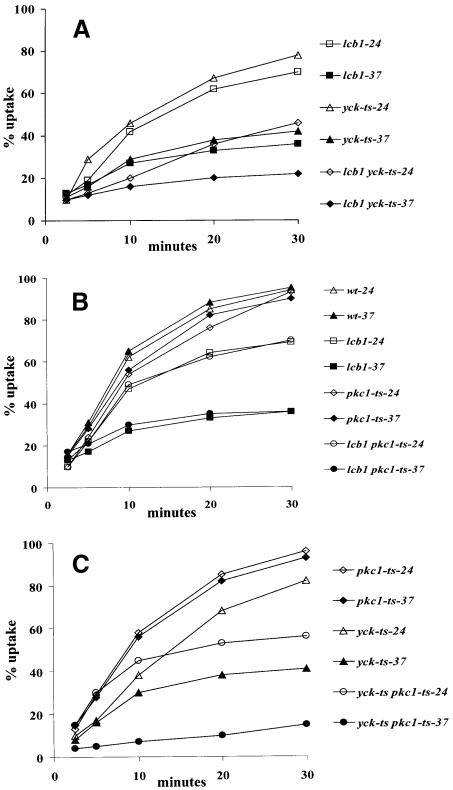
To investigate further the relationship between Yckp functions and sphingoid base synthesis, we tested for genetic interactions between the lcb1 and yck mutations. YCK1 and YCK2 have an overlapping function for cell growth (Robinson et al., 1992). Preliminary experiments showed that they also have an overlapping function in endocytosis, because single yck1Δ or yck2Δ mutant strains showed wild-type internalization (data not shown). Therefore, we crossed a yck-ts mutant (yck1Δ yck2-2-ts) with the lcb1-100 mutant to create a yck-ts lcb1-100 mutant (yck1Δ yck2-2-ts lcb1-100) that is conditional for both casein kinase I activity and sphingoid base synthesis. Next, we measured α-factor internalization in these strains at 24 and 37°C (Figure 5A). At 24°C, yck-ts cells internalized α-factor rapidly and to a similar extent to lcb1-100 cells. In contrast, yck-ts lcb1-100 cells showed a strongly reduced rate of α-factor internalization at 24°C (Figure 5A). Consistent with previous reports (Hicke et al., 1998), the yck-ts mutant showed a defect in α-factor internalization at 37°C, because the Yck kinases are required for phosphorylation of the α-factor receptor (Hicke et al., 1998). Phosphorylation and ubiquitylation of the receptor are required for internalization. At 37°C the yck-ts lcb1-100 cells were even more defective in α-factor uptake than either yck-ts or lcb1-100 strains (Figure 5A). These results reveal an enhancement of the endocytic defect at both permissive and restrictive temperature when lcb1-100 and yck-ts mutations are combined, suggesting that the two functions may be interconnected for the internalization step of endocytosis.
Fig. 5. (A) lcb1-100 and yck-ts mutations have a synthetic effect on endocytosis. The single mutant lcb1-100 (RH3802) and yck-ts (RH4336) cells were assayed for α-factor uptake at both 24°C (open symbols) and 37°C (closed symbols) and compared with the double mutant lcb1 yck-ts (RH4337) cells. (B) Radiolabeled α-factor uptake assays were performed at 24°C (open symbols) or 37°C (closed symbols) on wild-type (RH448), pkc1-ts (RH4325), lcb1-100 (RH3802) and lcb1 pkc1-ts (RH4329) strains. (C) Internalization assays were performed at 24°C (open symbols) and 37°C (closed symbols) on pkc1-ts (RH4325), yck-ts (RH4742) and yck-ts pkc1-ts (RH4598) mutants.
Loss of Pkc1p activity does not affect endocytosis
As shown above, overexpression of PKC1 restores the lcb1-100 defect in endocytosis. To determine whether Pkc1p kinase activity is required for the internalization step of endocytosis and whether the two genes LCB1 and PKC1 showed a genetic interaction, endocytosis of pkc1-2-ts and lcb1-100 pkc1-2-ts strains was assayed (Figure 5B). The pkc1-2-ts strain harbors a chromosomal deletion of the PKC1 gene (pkc1::LEU2) and is kept alive by a plasmid-borne temperature-sensitive pkc1-2-ts mutant allele. The single and double mutant strains were assayed for α-factor uptake at 24 and 37°C and compared with wild-type cells (Figure 5B). The pkc1-2-ts mutant cells internalized α-factor with the same rate as wild-type cells at 24 and 37°C, and the lcb1-100 pkc1-2-ts strain showed the same levels of internalization as lcb1-100 cells at both 24 and 37°C (Figure 5B). Therefore, we conclude that the loss of Pkc1p kinase activity does not affect the internalization step of endocytosis and does not increase the lcb1-100 defect in endocytosis at permissive or restrictive temperature.
Yck2p and Pkc1p have overlapping function in endocytosis
To determine whether Pkc1p and Yck2p have an overlapping role in endocytosis, we assayed α-factor uptake in single (pkc1-2-ts and yck-ts) and double (pkc1-ts yck-ts) mutant strains at 24 and 37°C. At 24°C, the pkc1-ts yck-ts strain showed a more severe α-factor internalization defect than either single mutant strain (Figure 5C). At 37°C, the double mutant strain was unable to internalize α-factor. The latter defect was again more severe than for either single mutant at this temperature.
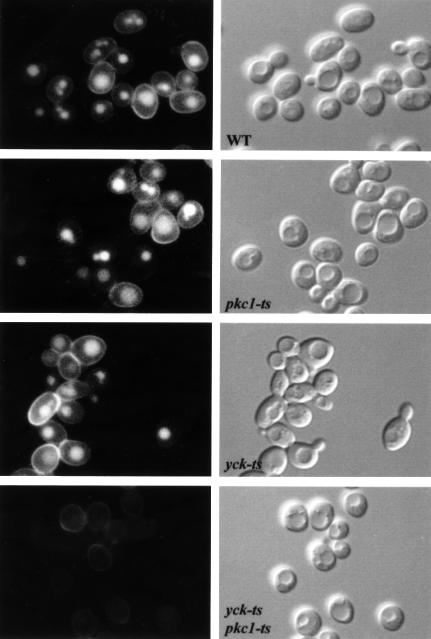
As the yck-ts mutant is defective for α-factor receptor phosphorylation it was important to examine whether the double mutant is also more severely defective for another endocytic marker. Therefore, we tested for a synthetic defect using the LY accumulation assay. LY accumulation in the single and double mutants was assayed at 37°C (Figure 6). Either single mutant accumulated LY in the vacuole to a similar extent to wild-type cells. This confirms the lack of an endocytic phenotype for the pkc1-2 mutant and suggests that the α-factor internalization defect in the yck-ts strain may be solely due to a lack of receptor modification, because there is no observable defect in LY accumulation. In contrast, the double mutant (pkc1-ts yck-ts) cells were completely defective in LY accumulation at 37°C (Figure 6). These results show that Pkc1p and Yck2p have an overlapping function in endocytosis, because only the cells with impaired activity for both kinases were defective for the internalization step of endocytosis.
Fig. 6. Yck and Pkc1p kinases have redundant activity in fluid-phase endocytosis. Wild-type (WT, RH448), pkc1-ts (RH4325), yck-ts (RH4742) and yck-ts pkc1-ts (RH4598) cells were incubated with LY at 37°C. To visualize LY uptake, cells were viewed by FITC-fluorescence optics (left panels). The same fields of cells were viewed by Nomarski optics (right panels) to visualize the vacuoles.
Overexpression of Yck2p or Pkc1p or inactivation of PP2A corrects the actin defect of the lcb1-100 mutant
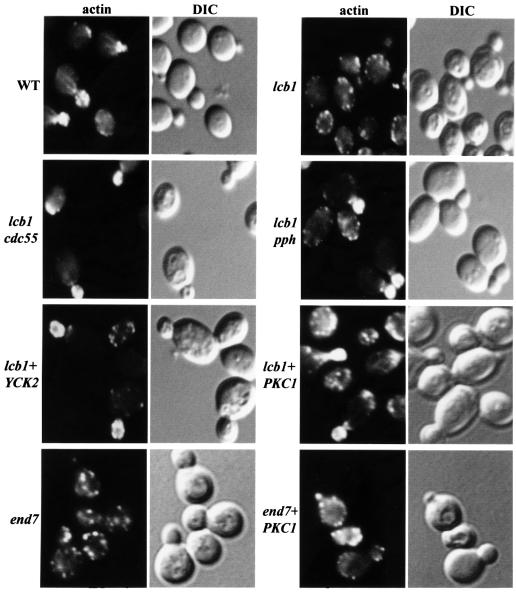
The lcb1-100 mutant is defective in the organization of the actin cytoskeleton at 37°C, but this defect and the endocytic defect can be corrected by addition of PHS or DHS to the lcb1-100 cells (Zanolari et al., 2000). Therefore, it is conceivable that overexpression of the kinases and inactivation of PP2A, which restored endocytosis by the lcb1-100 mutant, may also correct the actin defect of the lcb1-100 mutant. To test this, we examined whether the loss of CDC55 and PPH21/22/3 function or Yck2p and Pkc1p overexpression in the lcb1-100 mutant could restore the polarized distribution of actin at 37°C. Wild-type, lcb1-100, lcb1-100 cdc55Δ, lcb1-100 pph-ts, lcb1-100+YEp-YCK2 and lcb1-100+YEp-PKC1 strains were grown at 24°C, shifted to 37°C for 2 h and the cells were fixed and stained with TRITC–phalloidin to visualize F-actin (Figure 7). A shift from 24 to 37°C causes a heat-induced reorganization of the actin cytoskeleton in wild-type yeast cells to a non-polarized distribution. Normal polarized actin localization is restored after 1.5–2 h at 37°C in wild-type cells (Figure 7). However, whereas the heat-induced reorganization of the actin cytoskeleton was transient in wild-type cells, this perturbation was irreversible in the lcb1-100 mutant cells, as seen by the accumulation of actin patches in the mother cell of the budded cells (Figure 7) (Zanolari et al., 2000). In contrast, lcb1-100 mutant cells that were suppressed for endocytosis either by PP2A mutations or by kinase overexpression, displayed a polarized distribution of actin that was similar to wild-type cells because cortical actin patches concentrated in the bud (Figure 7). Furthermore, PKC1 overexpression in lcb1-100 cells also restored the actin cables (Figure 7).
Fig. 7. Loss of function of P2A or kinase overexpression suppresses the lcb1-100 actin organization defect. Logarithmic cultures of wild-type (WT, RH448), lcb1 (RH3802), lcb1 cdc55 (RH3807), lcb1 pph-ts (RH4191) and lcb1-100 (RH3802) cells with high copy number plasmid containing either YCK2 or PKC1 genes (lcb1+YCK2 or lcb1+PKC1) and end7-1 (act1, RH2069) cells with or without the PKC1 plasmid (end7+PKC1) were grown at 24°C, shifted to 37°C for 2 h, fixed, stained with TRITC–phalloidin and observed by fluorescence (actin) and Nomarski (DIC) microscopy.
PKC1 has been shown to mediate signaling to the actin cytoskeleton in yeast (Helliwell et al., 1998b). To determine whether the suppression by PKC1 was specific for the sphingoid base synthesis requirement of lcb1-100 cells, another endocytic mutant that displays actin cytoskeleton defects, end7-1 (act1) (Munn et al., 1995), was transformed by the YEp-PKC1 plasmid and assayed for actin localization (Figure 7). Proper actin localization was not restored by PKC1 overexpression in this mutant, showing that suppression is specific for sphingoid base synthesis. The above results suggest that one target of the sphingoid base synthesis requirement is likely to be the actin cytoskeleton, because overexpression of the kinases and mutation of the PP2A subunits specifically corrected the actin defect in the lcb1-100 mutant.
Discussion
The major finding of this study is that overexpression of specific kinases or loss of PP2A activity suppresses the sphingoid base synthesis requirement for both endocytosis and actin cytoskeleton organization in yeast. Interestingly, neither the protein kinase overexpression nor the PP2A inactivation were able to suppress the temperature- sensitive growth phenotype displayed by the lcb1-100 mutant (data not shown). Moreover, the loss of PP2A activity did not restore the ceramide synthesis defect observed in the lcb1-100 mutant (Zanolari et al., 2000), since the lcb1-100 strain and the double lcb1-100 cdc55Δ mutant showed the same reduction in sphingolipid synthesis at 37°C when compared with the wild-type strain or with the cdc55Δ mutant (C.Sütterlin and H.Riezman, unpublished data). Therefore, the suppressor effect was specific for the lcb1-100 endocytic and actin defects. These results suggest that sphingoid bases could act by activating protein kinases that are required for the internalization step of endocytosis. Two likely candidates for this activation are Yck2p and Pkc1p, which have an overlapping function in endocytosis. The ultimate target of sphingoid base is likely to be the endocytic machinery and/or the actin cytoskeleton.
We demonstrated that loss of CDC55 or PPH21/22/3 function completely suppressed the endocytic defect present in the lcb1-100 mutant strain. On the other hand, loss of SIT4 activity had no effect on endocytosis in the lcb1-100 strain. Previous studies have suggested that a yeast PP2A could be activated by C2-ceramide that induces a G1 arrest of cells (Fishbein et al., 1993; Nickels and Broach, 1996). Yeast CAPP was proposed to be composed of two regulatory subunits, Cdc55p and Tpd3p, and a major catalytic subunit, Sit4p (Nickels and Broach, 1996). Our results suggest a common function for Cdc55p and Pph21p/Pph22p/Pph3p, but not Sit4p, as a possible antagonist of endocytosis. Other studies have shown a shared function between Cdc55p and Pph21p/Pph22p/Pph3p (Sneddon et al., 1990; Ronne et al., 1991). Interestingly, there is some evidence for partial redundant function between SIT4 and PPH22. Elevated gene dosage of PPH22 partially suppressed the sit4-102 mutation (Sutton et al., 1991). Biochemical data suggest that Tpd3p and Cdc55p interact with Pph21p/Pph22p in cells. However, it is possible that these two regulatory subunits associate with a variety of different catalytic subunits, including Pph21p/Pph22p/Pph3p or Sit4p, for different functions, some activated by lipids and others not. Our study suggests that PP2A activity acts in opposition to the sphingoid base synthesis requirement in endocytosis. However, our study provides no information on the potential lipid activation of PP2A.
The fact that the loss of PP2A activity completely abrogated the sphingoid base synthesis requirement for endocytosis suggests that the major role of sphingoid base synthesis in endocytosis is to stimulate protein phosphorylation. A similar suppression by overexpression of YCK2 or PKC1 supports this hypothesis. The suppression is specific for these two kinase genes because overexpression of other kinase genes, some even belonging to the same kinase families, did not suppress the lcb1-100 endocytic defect. Overexpression of CMK2, encoding a kinase that is activated by calmodulin, had no effect on the lcb1-100 endocytic defect, even though the internalization step of endocytosis in yeast is dependent on calmodulin (Kübler et al., 1994; Geli et al., 1998). Yck1p, a kinase belonging to the same family of casein kinase I isoforms and 66% identical in amino acid sequence to Yck2p (Robinson et al., 1992), did not suppress lcb1-100, suggesting that some specificity, in either expression or substrate selection, is required to suppress lcb1-100 for endocytosis. Yck1p and Yck2p have previously been implicated in endocytosis, due to their requirement for internalization of both a- and α-factor receptors (Panek et al., 1997; Hicke et al., 1998) and for down-regulation of the uracil permease, Fur4p (Marchal et al., 1998). It has been demonstrated that casein kinase I activity is required for ligand-induced phosphorylation and subsequent ubiquitylation of the α-factor receptor leading to its internalization (Hicke et al., 1998). The mode of action of Yck2p in suppression of lcb1-100 is different because α-factor receptor phosphorylation is not defective in the lcb1-100 mutant (Zanolari et al., 2000). On the other hand, Yck2p function is likely to be associated with the sphingoid base synthesis requirement for endocytosis, because the lcb1-100 and yck-ts mutants had an additive effect on endocytosis at both permissive and restrictive temperature.
An increased gene dosage of PKC1 or expression of a dominant, activated allele of PKC1 suppressed the endocytic defect of the lcb1-100 mutant, suggesting that Pkc1p is required in its active form for this suppression. The Ypk1p kinase that has 44–46% identity to protein kinase C isozymes in the catalytic domain did not suppress the lcb1-100 endocytic defect, suggesting that specificity in the substrate selection is required for this suppression. It has been shown that the kinase activity of an atypical mammalian PKC isozyme, PKC ζ, is regulated by sphingosine and by ceramide (Muller et al., 1995). This PKC ζ is not activated by Ca2+ or DAG like the classical PKCs (Ono et al., 1989). Interestingly, yeast Pkc1p has a similar pattern of activation, meaning that Pkc1p does not respond to Ca2+ or to DAG (Antonsson et al., 1994). It has been proposed that ceramide activates an atypical protein kinase C, Raf-1 and KSR kinases through binding to their cysteine-rich domains (CRDs) (van Blitterswijk, 1998). Sequence comparison of the CRDs revealed that all these ceramide-activated proteins share conserved Cys/His residues in their CRDs, but lack several residues known to be important for phorbol ester/DAG binding (van Blitterswijk, 1998). Interestingly, the sequence comparison of the C1-domain of the yeast Pkc1p with the CRDs of these proteins showed that yeast Pkc1p also lacks several of the residues essential for phorbol ester/DAG binding (data not shown).
In yeast, Pkc1p activates a MAP kinase cascade that is composed of several kinases, Bck1p, Mkk1p/Mkk2p and Mpk1p, acting sequentially in order to regulate the dynamics of the cell wall and actin cytoskeleton organization (Lee and Levin, 1992; Irie et al., 1993; Lee et al., 1993; Nonaka et al., 1995; Helliwell et al., 1998). None of the kinases that belong to this cascade was able to restore the endocytic defect of the lcb1-100 mutant. This result suggests that the suppressor effect of Pkc1p overexpression is not mediated through the known Pkc1p MAP kinase signaling pathway. It has been shown previously that deletion of kinases of this cascade results in phenotypes that are less severe than that of PKC1 deleted strains, also suggesting the existence of other pathways downstream of Pkc1p (Irie et al., 1993; Mazzoni et al., 1993). Moreover, a recent study showed that the transient depolarization of the actin cytoskeleton in response to environmental stress was controlled by a signaling pathway consisting of PKC1 and an as yet unidentified PKC1 effector branch (Delley and Hall, 1999).
It is interesting to note that YCK2 overexpression suppressed the α-factor internalization defect of lcb1-100 better than PKC1 overexpression, whereas the opposite was true for the LY accumulation defect. This could be due to the nature of the assays. First, it should be noted that while the α-factor internalization assay apparently measures a single trafficking event, the internalization step of endocytosis, there are prerequisites for this step, including receptor phosphorylation and ubiquitylation. One effect that could contribute to better suppression by YCK2 in this assay is its possible function to stimulate receptor phosphorylation. Secondly, the LY accumulation assay measures a series of events leading to and from the endocytic pathway and the vacuole. A possible explanation for an apparently better suppression by PKC1 rather than YCK2 overexpression would be if PKC1 overexpression somehow inhibited another step of the pathway, such as LY recycling.
The lcb1-100 mutant is defective in the uptake step of endocytosis and in the organization of the actin cytoskeleton at 37°C, and these defects can be suppressed by addition of PHS or DHS to the cells (Zanolari et al., 2000). As shown previously, the actin cytoskeleton plays an essential role in the internalization step of endocytosis in yeast, because yeast mutants in actin and actin-binding proteins are defective in endocytosis (Kübler and Riezman, 1993; Munn et al., 1995). Here we showed that overexpression of Yck2p or Pkc1p or mutations in PP2A restored the actin cytoskeleton organization defect of the lcb1-100 mutant. It is interesting to note that neither YCK2 nor PKC1 overexpression restored the endocytic defect of other mutants that are defective in the internalization step of endocytosis and in actin cytoskeleton organization, indicating that this suppressor effect was specific for the lcb1-100 mutation. Moreover, overexpression of the lipid kinase Mss4p, a phosphatidylinositol-4-phosphate 5-kinase that controls actin cytoskeleton organization in yeast (Desrivières et al., 1998), was not able to restore the endocytic (Figure 2A) or the actin (data not shown) defects of the lcb1-100 cells.
Even though a yck-ts strain is defective for both a- and α-factor receptor internalization, we have shown here that the strain does not have a general endocytic defect, because fluid-phase endocytosis accumulation of LY in its vacuole is normal at non-permissive temperature. This suggests that the defect in receptor-mediated endocytosis is most likely due to inefficient receptor phosphorylation and ubiquitylation. The pkc1-ts mutant was not defective for fluid-phase endocytosis either. Only cells with impaired activity of both Yck2p and Pkc1p kinases were defective in both receptor-mediated and fluid-phase endocytosis, suggesting a functional redundancy. What could be this overlapping function? Recent work has shown that Yck2p kinase displays plasma membrane localization that is dynamic during the cell cycle and coincides with redistribution of actin structures (Robinson et al., 1999). Pkc1p is known to function in actin cytoskeleton organization. It is possible that a function, shared between the two kinases in actin dynamics, is required for endocytic internalization. This cannot simply be polarized actin distribution, because several mutants, including pph-ts (Lin and Arndt, 1995), show delocalized actin without affecting endocytosis (Figure 1).
It is interesting that the requirement for sphingoid base can be suppressed either by overexpression of YCK2 or loss of CDC55 activity. In other cases, mutations in these two activities lead to very similar phenotypes (Healy et al., 1991; Robinson et al., 1993), and the combination between cdc55Δ and yck-ts is lethal, suggesting a functional relationship between Yck2p activity and PP2A activity (Robinson et al., 1993). For endocytosis, their activities may act antagonistically on the same substrate, but other explanations are possible. It has been reported recently that some protein kinases and protein phosphatases form a protein complex, in which the two activities may regulate each other (Camps et al., 1998; Westphal et al., 1998). It could be that loss of Cdc55p would cause an increase in Yck2p (or Pkc1p) activity. If these two proteins do form such a complex an interesting possibility arises. Small molecules, for instance sphingoid bases, may regulate the relative phosphorylation/dephosphorylation activity of the complex.
In summary, our results are the first example of sphingoid base synthesis being used to control protein phosphorylation regulating a step of membrane trafficking. Many details remain to be discovered, including the demonstration and identification of a sphingoid base-activated protein kinase and the mechanism whereby sphingoid base controls the relative activities of protein kinases and phosphatases. The ease of genetic and molecular studies in yeast should help to understand these questions.
Materials and methods
Plasmids, strains, media and genetic manipulations
Plasmids and yeast strains used in this study are listed in Tables I and II, respectively. Yeast strain RH3745 was constructed by crossing AHY86 (MATa cdc55::LEU2 leu2 ura3 his3; Healy et al., 1991) twice into our genetic background. All other disruption mutants were created by integrative transformation using standard techniques. To construct the temperature-sensitive protein phosphatase strains, heterozygous disruptions were made in a diploid strain, and after introduction of the relevant temperature-sensitive allele on a plasmid the diploids were sporulated and dissected to generate the MATa strains used in this study. Disruption plasmids pMC101 (pph21::HIS3), pMC104 (pph22::LEU2), pMC89 (pph3::URA3) for the PPH genes were from H.Ronne (Uppsala) and the sit4::HIS3 disruption cassette was amplified by PCR from strain Y1361 (Nickels and Broach, 1996). Plasmids with temperature-sensitive protein phosphatase alleles were CB3138 (pph21-102/TRP1/CEN; Lin and Arndt, 1995) and CB213 (sit4-102/LEU2/CEN; Sutton et al., 1991). Yeast cell cultures and genetic manipulations were carried out essentially as described by Sherman et al. (1983). Yeast cells were transformed by the lithium acetate method using single-stranded carrier DNA and dimethyl sulfoxide (DMSO) (Schiestl and Gietz, 1989; Hill et al., 1991). Rich YPUAD medium and synthetic minimal media (SD) complemented with the appropriate nutrients for plasmid maintenance were prepared as described (Munn et al., 1995).
Table II. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| RH448 | leu2 ura3 his4 lys2 | laboratory strain |
| RH1597 | leu2 ura3 his4 end4-1 | Raths et al. (1993) |
| RH1800 | leu2 ura3 his4 | laboratory strain |
| RH2069 | leu2 ura3 his4 end7-1 | Munn et al. (1995) |
| RH2077 | leu2 ura3 his4 end5-1 | Munn et al. (1995) |
| RH2082 | leu2 ura3 his4 end6-1 | Munn et al. (1995) |
| RH3745 | leu2 ura3 ade2 cdc55::LEU2 | this study |
| RH3802 | leu2 ura3 his4 his3 ade2 lys2 lcb1-100 | this study |
| RH3807 | leu2 ura3 his4 ade2 lys2 lcb1-100 cdc55::LEU2 | this study |
| RH3809 | leu2 ura3 his4 lcb1-100 | this study |
| RH4137 | leu2 ura3 his3 lys2 trp1 pph21::HIS3 pph22::LEU2 pph3::URA3 (CB3138[pph21–102]) | this study |
| RH4183 | leu2 ura3 his3 lys2 trp1 sit4::HIS3 (CB213[sit4-102]) | this study |
| RH4191 | leu2 ura3 his3 lys2 trp1 lcb1-100 pph21::HIS3 pph22::LEU2 pph3:: URA3 (CB3138[pph21–102]) | this study |
| RH4195 | leu2 ura3 his3 his4 ade2 trp1 lcb1-100 sit4::HIS3 (CB213[sit4-102]) | this study |
| RH4325 | leu2 ura3 his4 lys2 trp1 pkc1::LEU2 (YCp50[pkc1-2]) | this study |
| RH4329 | leu2 ura3 his4 lys2 trp1 lcb1-100 pkc1::LEU2 (YCp50[pkc1-2]) | this study |
| RH4336 | leu2 ura3 trp1 yck1Δ yck2-2 | this study |
| RH4337 | leu2 ura3 his3 ade2 trp1 lcb1-100 yck1Δ yck2-2 | this study |
| RH4598 | leu2 ura3 his3 lys2 ade2 pkc1::LEU2 (YCp50[pkc1-2]) yck1Δ yck2-2 | this study |
| RH4742 | leu2 ura3 his3 ade2 lys2 yck1Δ yck2-2 | this study |
All strains listed in this table are MATa and bar1.
Endocytosis assays
LY (Fluka, Buchs, Switzerland) assays were performed as described (Dulic et al., 1991; Munn and Riezman, 1994). Yeast pre-cultures were grown at 24°C in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were then grown at 24°C in YPUAD to mid-log phase, shifted to 37°C for 15 min and incubated for 1 h at 37°C with LY. [35S]α-factor uptake assays were performed on mid-log phase cells using the continuous presence protocol as described (Dulic et al., 1991). Pre-cultures were performed at 24°C in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were then grown at 24°C in YPUAD medium; the α-factor uptake assays were carried out at 24 or 37°C after 15 min pre-incubation at the respective temperature. All uptake assays were performed at least twice, the results shown are from one of the independent experiments that gave nearly identical results.
Rhodamine–phalloidin staining of actin
Yeast cell pre-cultures were grown at 24°C in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were then grown at 24°C in YPUAD medium to early log phase. Cells at 1 × 107 cells/ml were then incubated for 2 h at 37°C, fixed in formaldehyde and stained with TRITC–phalloidin (Sigma, St Louis, MO) to visualize F-actin essentially as described previously (Benedetti et al., 1994).
Acknowledgments
Acknowledgements
We thank L.Robinson, K.Arndt, J.Pringle, H.Ronne, M.Stark, T.Beck, T.Schmelzle and S.Desrivières for strains and plasmids, Jeannette Holenstein and Thomas Aust for technical assistance, C.Sütterlin for communicating unpublished results, and Antje Heese-Peck and Pierre Morsomme for helpful comments on the manuscript. This work was supported by the Canton of Basel-Stadt, EMBO and HFSP long-term fellowships (to S.F.) and a grant from the Swiss National Science Foundation (to H.R.).
References
- Anderson R.G. (1998) The caveolae membrane system. Annu. Rev. Biochem., 67, 199–225. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit,S., Friedli,L., Payton,M.A. and Paravicini,G. (1994) Protein kinase C in yeast. Characteristics of the Saccharomyces cerevisiae PKC1 gene product. J. Biol. Chem., 269, 16821–16828. [PubMed] [Google Scholar]
- Bankaitis V.A., Aitken,J.R., Cleves,A.E. and Dowhan,W. (1990) An essential role for a phospholipid transfer protein in yeast Golgi function. Nature, 347, 561–562. [DOI] [PubMed] [Google Scholar]
- Benedetti H., Raths,S., Crausaz,F. and Riezman,H. (1994) The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell, 5, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G.M., Reilly,A.M., Daniels,R.H., King,C.C., Olivera,A., Spiegel,S. and Knaus,U.G. (1998) A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J. Biol. Chem., 273, 8137–8144. [DOI] [PubMed] [Google Scholar]
- Camps M., Nichols,A., Gillieron,C., Antonsson,B., Muda,M., Chabert,C., Boschert,U. and Arkinstall,S. (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science, 280, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Chen P., Lee,K.S. and Levin,D.E. (1993) A pair of putative protein kinase genes (YPK1 and YPK2) is required for cell growth in Sacharomyces cerevisiae. Mol. Gen. Genet., 236, 443–447. [DOI] [PubMed] [Google Scholar]
- Cuvillier O., Pirianov,G., Kleuser,B., Vanek,P.G., Coso,O.A., Gutkind,S. and Spiegel,S. (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature, 381, 800–803. [DOI] [PubMed] [Google Scholar]
- Delley P.A. and Hall,M.N. (1999) Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol., 147, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivières S., Cooke,F.T., Parker,P.J. and Hall,M.N. (1998) MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem., 273, 15787–15793. [DOI] [PubMed] [Google Scholar]
- Dickson R.C., Nagiec,E.E., Skrzypek,M., Tillman,P., Wells,G.B. and Lester,R.L. (1997) Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem., 272, 30196–30200. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R.T., Kamibayashi,C., Mumby,M.C. and Hannun,Y.A. (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem., 268, 15523–15530. [PubMed] [Google Scholar]
- Dulic V., Egerton,M., Elguindi,I., Raths,S., Singer,B. and Riezman,H. (1991) Yeast endocytosis assays. Methods Enzymol., 194, 697–710. [DOI] [PubMed] [Google Scholar]
- Fishbein J.D., Dobrowsky,R.T., Bielawska,A., Garrett,S. and Hannun,Y.A. (1993) Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J. Biol. Chem., 268, 9255–9261. [PubMed] [Google Scholar]
- Geli M.I. and Riezman,H. (1998) Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci., 111, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Geli M.I., Wesp,A. and Riezman,H. (1998) Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. EMBO J., 17, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y.A. (1994) The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem., 269, 3125–3128. [PubMed] [Google Scholar]
- Hannun Y.A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science, 274, 1855–1859. [DOI] [PubMed] [Google Scholar]
- Hannun Y.A., Loomis,C.R., Merrill,A.H.,Jr and Bell,R.M. (1986) Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem., 261, 12604–12609. [PubMed] [Google Scholar]
- Healy A.M., Zolnierowicz,S., Stapleton,A.E., Goebl,M., DePaoli-Roach,A.A. and Pringle,J.R. (1991) CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol., 11, 5767–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S.B., Howald,I., Barbet,N. and Hall,M.N. (1998a) TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics, 148, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S.B., Schmidt,A., Ohya,Y. and Hall,M.N. (1998b) The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol., 8, 1211–1214. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. (1995) MAP kinase pathways in yeast: for mating and more. Cell, 80, 187–197. [DOI] [PubMed] [Google Scholar]
- Hicke L., Zanolari,B. and Riezman,H. (1998) Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J. Cell Biol., 141, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Donald,K.A., Griffiths,D.E. and Donald,G. (1991) DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res., 19, 5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A., Sütterlin,C., Manning-Krieg,U., Movva,N.R. and Riezman,H. (1994) Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J., 13, 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. and Plowman,G.D. (1997) The protein kinases of budding yeast: six score and more. Trends Biochem. Sci., 22, 18–22. [DOI] [PubMed] [Google Scholar]
- Irie K., Takase,M., Lee,K.S., Levin,D.E., Araki,H., Matsumoto,K. and Oshima,Y. (1993) MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol., 13, 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S. and Hannun,Y.A. (1996) Ceramide: role in growth inhibitory cascades. J. Lipid Mediat. Cell Signal., 14, 295–301. [DOI] [PubMed] [Google Scholar]
- Jenkins G.M., Richards,A., Wahl,T., Mao,C., Obeid,L. and Hannun,Y. (1997) Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem., 272, 32566–32572. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Jung,U.S., Piotrowski,J. and Levin,D.E. (1995) The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev., 9, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kearns B.G., McGee,T.P., Mayinger,P., Gedvilaite,A., Phillips,S.E., Kagiwada,S. and Bankaitis,V.A. (1997) Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature, 387, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.C., Zenke,F.T., Dawson,P.E., Dutil,E.M., Newton,A.C., Hemmings,B.A. and Bokoch,G.M. (2000) Sphingosine is a novel activator of 3-phosphoinositide- dependent kinase-1. J. Biol. Chem., 29, in press. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Gu,F. and Gruenberg,J. (1998) Lipids, lipid domains and lipid–protein interactions in endocytic membrane traffic. Semin. Cell Dev. Biol., 9, 517–526. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. and Hannun,Y.A. (1999) Ceramide and apoptosis. Trends Biochem. Sci., 24, 224–225. [DOI] [PubMed] [Google Scholar]
- Kübler E. and Riezman,H. (1993) Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J., 12, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E., Schimmöller,F. and Riezman,H. (1994) Calcium-independent calmodulin requirement for endocytosis in yeast. EMBO J., 13, 5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S. and Levin,D.E. (1992) Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol., 12, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Irie,K., Gotoh,Y., Watanabe,Y., Araki,H., Nishida,E., Matsumoto,K. and Levin,D.E. (1993) A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol., 13, 3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.C. and Arndt,K.T. (1995) The role of Saccharomyces cerevisiae type 2A phosphatase in the actin cytoskeleton and in entry into mitosis. EMBO J., 14, 2745–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S.M., Thornton,R., Tu,Z., Kurtz,M.B., Nickels,J., Broach,J., Menzeleev,R. and Spiegel,S. (1998) Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl Acad. Sci. USA, 95, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Saba,J.D. and Obeid,L.M. (1999) The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J., 342, 667–675. [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Haguenauer-Tsapis,R. and Urban-Grimal,D. (1998) A PEST-like sequence mediates phosphorylation and efficient ubiquitination of yeast uracil permease. Mol. Cell. Biol., 18, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C., Zarov,P., Rambourg,A. and Mann,C. (1993) The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol., 123, 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald O.B., Hannun,Y.A., Reynolds,C.H. and Sahyoun,N. (1991) Activation of casein kinase II by sphingosine. J. Biol. Chem., 266, 21773–21776. [PubMed] [Google Scholar]
- Muller G., Ayoub,M., Storz,P., Rennecke,J., Fabbro,D. and Pfizenmaier,K. (1995) PKCζ is a molecular switch in signal transduction of TNF-α, bifunctionally regulated by ceramide and arachidonic acid. EMBO J., 14, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L. and Riezman,H. (1994) Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J. Cell Biol., 127, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Stevenson,B.J., Geli,M.I. and Riezman,H. (1995) end5, end6 and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell, 6, 1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Heese-Peck,A., Stevenson,B.J., Pichler,H. and Riezman,H. (1999) Specific sterols required for the internalization step of endocytosis in yeast. Mol. Biol. Cell, 10, 3943–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec M.M., Baltisberger,J.A., Wells,G.B., Lester,R.L. and Dickson,R.C. (1994) The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl Acad. Sci. USA, 91, 7899–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J.T. and Broach,J.R. (1996) A ceramide-activated protein phosphatase mediates ceramide-induced G1 arrest of Saccharomyces cerevisiae. Genes Dev., 10, 382–394. [DOI] [PubMed] [Google Scholar]
- Nonaka H., Tanaka,K., Hirano,H., Fujiwara,T., Kohno,H., Umikawa,M., Mino,A. and Takai,Y. (1995) A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J., 14, 5931–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii,T., Ogita,K., Kikkawa,U., Igarashi,K. and Nishizuka,Y. (1989) Protein kinase C ζ subspecies from rat brain: its structure, expression and properties. Proc. Natl Acad. Sci. USA, 86, 3099–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek H.R., Stepp,J.D., Engle,H.M., Marks,K.M., Tan,P.K., Lemmon,S.K. and Robinson,L.C. (1997) Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J., 16, 4194–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkareva M., Khan,W.A., Alessenko,A.V., Sahyoun,N. and Hannun,Y.A. (1992) Sphingosine activation of protein kinases in Jurkat T cells. In vitro phosphorylation of endogenous protein substrates and specificity of action. J. Biol. Chem., 267, 15246–15251. [PubMed] [Google Scholar]
- Pushkareva M., Bielawska,A., Menaldiv,D., Liotta,D. and Hannun,Y.A. (1993) Regulation of sphingosine-activated protein kinases: selectivity of activation by sphingoid bases and inhibition by non-esterified fatty acids. Biochem. J., 294, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S., Rohrer,J., Crausaz,F. and Riezman,H. (1993) end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol., 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. (1998) Down regulation of yeast G protein-coupled receptors. Semin. Cell Dev. Biol., 9, 129–134. [DOI] [PubMed] [Google Scholar]
- Riezman H., Munn,A., Geli,M.I. and Hicke,L. (1996) Actin-, myosin- and ubiquitin-dependent endocytosis. Experientia, 52, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Robinson L.C. et al. (1992) Yeast casein kinase I homologues: an essential gene pair. Proc. Natl Acad. Sci. USA, 89, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.C., Menold,M.M., Garrett,S. and Culbertson,M.R. (1993) Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol. Cell. Biol., 13, 2870–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.C., Bradley,C., Bryan,J.D., Jerome,A., Kweon,Y. and Panek,H.R. (1999) The yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol. Biol. Cell, 10, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S.K., Skretting,G., Garred,O., Vilhardt,F., van Deurs,B. and Sandvig,K. (1999) Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell, 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne H., Carlberg,M., Hu,G.Z. and Nehlin,J.O. (1991) Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol. Cell. Biol., 11, 4876–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R.H. and Gietz,R.D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet., 16, 339–346. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Beck,T., Koller,A., Kunz,J. and Hall,M.N. (1998) The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J., 17, 6924–6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu P.V., Takegawa,K., Fry,M.J., Stack,J.H., Waterfield,M.D. and Emr,S.D. (1993) Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science, 260, 88–91. [DOI] [PubMed] [Google Scholar]
- Sherman F., Fink,G. and Hicks,J.B. (1983) Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Skrzypek M.S., Nagiec,M.M., Lester,R.L. and Dickson,R.C. (1999) Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol., 181, 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon A.A., Cohen,P.T. and Stark,M.J. (1990) Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J., 9, 4339–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A., Gaidarov,I., Kobylarz,K., Lampson,M.A., Keen,J.H. and McGraw,T.E. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl Acad. Sci. USA, 96, 6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Immanuel,D. and Arndt,K.T. (1991) The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol., 11, 2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W.J. (1998) Hypothesis: ceramide conditionally activates atypical protein kinases C, Raf-1 and KSR through binding to their cysteine-rich domains. Biochem. J., 331, 679–680. [PMC free article] [PubMed] [Google Scholar]
- van Zyl W., Huang,W., Sneddon,A.A., Stark,M., Camier,S., Werner,M., Marck,C., Sentenac,A. and Broach,J.R. (1992) Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol. Cell. Biol., 12, 4946–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hoekstra,M.F., DeMaggio,A.J., Dhillon,N., Vancura,A., Kuret,J., Johnston,G.C. and Singer,R.A. (1996) Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol. Cell. Biol., 16, 5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal R.S., Anderson,K.A., Means,A.R. and Wadzinski,B.E. (1998) A signaling complex of Ca2+-calmodulin-dependent protein kinase IV and protein phosphatase 2A. Science, 280, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Wolff R.A., Dobrowsky,R.T., Bielawska,A., Obeid,L.M. and Hannun,Y.A. (1994) Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J. Biol. Chem., 269, 19605–19609. [PubMed] [Google Scholar]
- Zanolari B., Friant,S., Funato,K., Sütterlin,C., Stevenson,B.J. and Riezman,H. (2000) Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J., 19, 2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]