Abstract
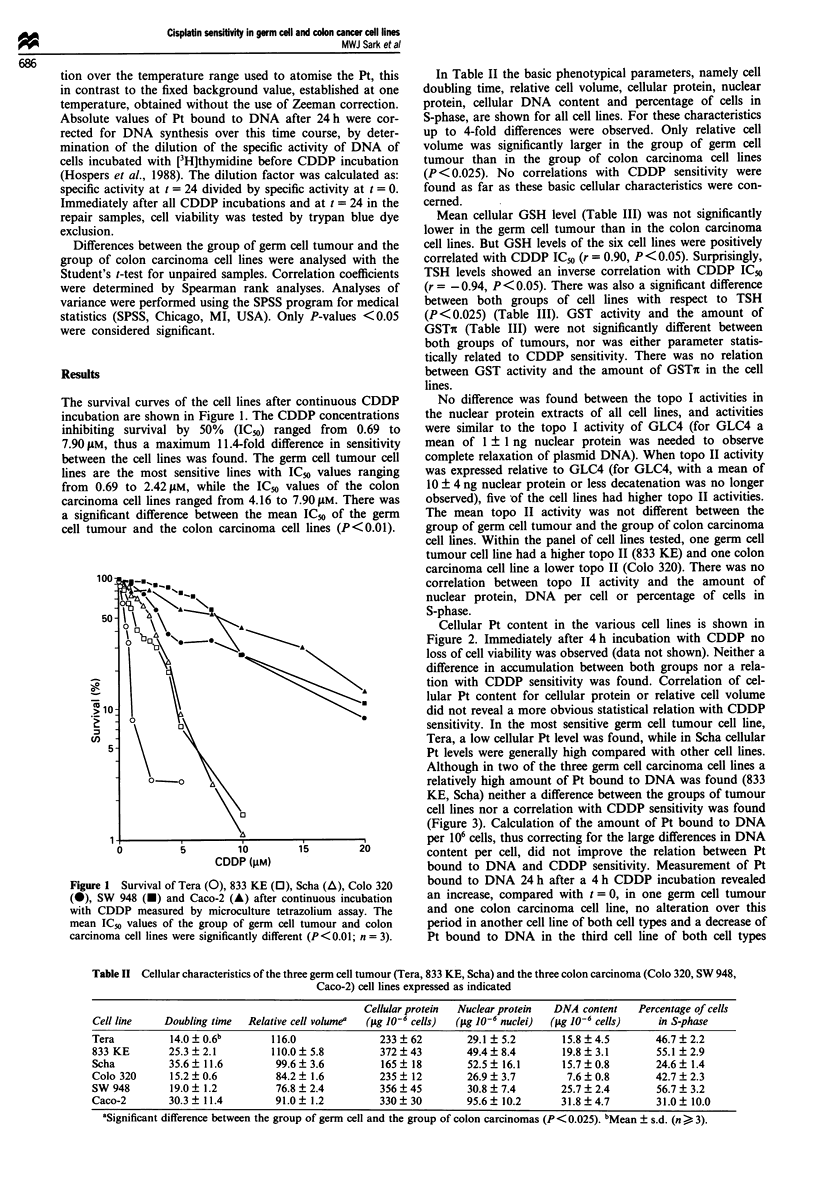
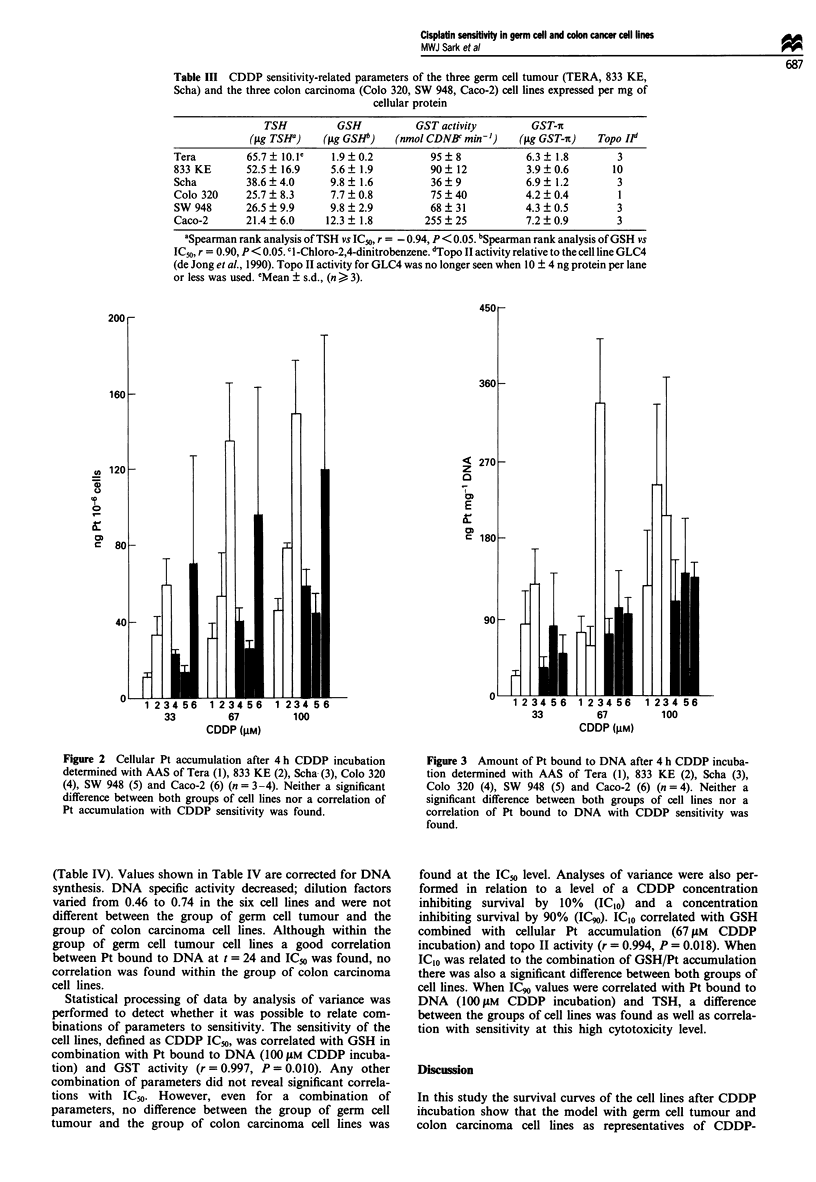
Cisplatin (CDDP) resistance mechanisms were studied in a model of three germ cell tumour and three colon carcinoma cell lines representing intrinsically CDDP-sensitive and -resistant tumours respectively. The CDDP sensitivity of the cell lines mimicked the clinical situation. The glutathione levels of the cell lines correlated with CDDP concentrations inhibiting cell survival by 50% (IC50); total cellular sulphydryl content (TSH) was unexpectedly inversely correlated with IC50. IC50 correlated neither with glutathione S-transferase (GST) nor with GST pi expression, topoisomerase I or II activity. Immediately after 4 h incubation with CDDP, platinum (Pt) accumulation and Pt bound to DNA were not correlated, but after another 24 h drug-free culture, Pt binding to DNA in germ cell tumour but not in colon carcinoma cell lines correlated with IC50. With the exception of in vitro sensitivity and TSH, none of the parameters studied discriminated between the two groups of cell lines. Correction of CDDP sensitivity parameters for phenotypical differences did not influence statistical correlations. Analysis of variance revealed a correlation between IC50 and the combination of glutathione, GST activity and Pt bound to DNA. But at other CDDP cytotoxicity levels sensitivity was also correlated with Pt accumulation, topoisomerase II activity and TSH in various combinations. This model of intrinsic CDDP resistance showed that multiple parameters ought to be studied to explain CDDP resistance, but did not elucidate the cause of the unique sensitivity of germ cell carcinoma, although the unexpected values of TSH deserve further attention.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Bedford P., Fichtinger-Schepman A. M., Shellard S. A., Walker M. C., Masters J. R., Hill B. T. Differential repair of platinum-DNA adducts in human bladder and testicular tumor continuous cell lines. Cancer Res. 1988 Jun 1;48(11):3019–3024. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Andrews P. W., Solter D., Cervenka J., Lange P. H., Fraley E. E. Cell line derived from a metastasis of a human testicular germ cell tumor. Cancer Res. 1980 Jul;40(7):2500–2506. [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Fram R. J., Woda B. A., Wilson J. M., Robichaud N. Characterization of acquired resistance to cis-diamminedichloroplatinum (II) in BE human colon carcinoma cells. Cancer Res. 1990 Jan 1;50(1):72–77. [PubMed] [Google Scholar]
- Fry A. M., Chresta C. M., Davies S. M., Walker M. C., Harris A. L., Hartley J. A., Masters J. R., Hickson I. D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991 Dec 15;51(24):6592–6595. [PubMed] [Google Scholar]
- Giaccone G., Gazdar A. F., Beck H., Zunino F., Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992 Apr 1;52(7):1666–1674. [PubMed] [Google Scholar]
- Hill B. T., Scanlon K. J., Hansson J., Harstrick A., Pera M., Fichtinger-Schepman A. M., Shellard S. A. Deficient repair of cisplatin-DNA adducts identified in human testicular teratoma cell lines established from tumours from untreated patients. Eur J Cancer. 1994;30A(6):832–837. doi: 10.1016/0959-8049(94)90301-8. [DOI] [PubMed] [Google Scholar]
- Hosking L. K., Whelan R. D., Shellard S. A., Bedford P., Hill B. T. An evaluation of the role of glutathione and its associated enzymes in the expression of differential sensitivities to antitumour agents shown by a range of human tumour cell lines. Biochem Pharmacol. 1990 Oct 15;40(8):1833–1842. doi: 10.1016/0006-2952(90)90364-q. [DOI] [PubMed] [Google Scholar]
- Hospers G. A., Mulder N. H., de Jong B., de Ley L., Uges D. R., Fichtinger-Schepman A. M., Scheper R. J., de Vries E. G. Characterization of a human small cell lung carcinoma cell line with acquired resistance to cis-diamminedichloroplatinum(II) in vitro. Cancer Res. 1988 Dec 1;48(23):6803–6807. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kelland L. R., Mistry P., Abel G., Freidlos F., Loh S. Y., Roberts J. J., Harrap K. R. Establishment and characterization of an in vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line. Cancer Res. 1992 Apr 1;52(7):1710–1716. [PubMed] [Google Scholar]
- Kelland L. R. The molecular basis of cisplatin sensitivity/resistance. Eur J Cancer. 1994;30A(6):725–727. doi: 10.1016/0959-8049(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Koropatnick J., Pearson J. Altered cisplatin and cadmium resistance and cell survival in Chinese hamster ovary cells expressing mouse metallothionein. Mol Pharmacol. 1993 Jul;44(1):44–50. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Masters J. R., Osborne E. J., Walker M. C., Parris C. N. Hypersensitivity of human testis-tumour cell lines to chemotherapeutic drugs. Int J Cancer. 1993 Jan 21;53(2):340–346. doi: 10.1002/ijc.2910530228. [DOI] [PubMed] [Google Scholar]
- McLaughlin K., Coren G., Masters J., Brown R. Binding activities of cis-platin-damage-recognition proteins in human tumour cell lines. Int J Cancer. 1993 Feb 20;53(4):662–666. doi: 10.1002/ijc.2910530423. [DOI] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Sluiter W. J., Meersma G. J., de Vries E. G. Relationship of cellular glutathione to the cytotoxicity and resistance of seven platinum compounds. Cancer Res. 1992 Dec 15;52(24):6885–6889. [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., de Vries E. G. The role of detoxifying systems in resistance of tumor cells to cisplatin and adriamycin. Cancer Treat Rev. 1990 Dec;17(4):389–407. doi: 10.1016/0305-7372(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Niimi S., Nakagawa K., Sugimoto Y., Nishio K., Fujiwara Y., Yokoyama S., Terashima Y., Saijo N. Mechanism of cross-resistance to a camptothecin analogue (CPT-11) in a human ovarian cancer cell line selected by cisplatin. Cancer Res. 1992 Jan 15;52(2):328–333. [PubMed] [Google Scholar]
- Oosterhuis J. W., Andrews P. W., Knowles B. B., Damjanov I. Effects of cis-platinum on embryonal carcinoma cell lines in vitro. Int J Cancer. 1984 Jul 15;34(1):133–139. doi: 10.1002/ijc.2910340123. [DOI] [PubMed] [Google Scholar]
- Parris C. N., Walker M. C., Masters J. R., Arlett C. F. Inherent sensitivity and induced resistance to chemotherapeutic drugs and irradiation in human cancer cell lines: relationship to mutation frequencies. Cancer Res. 1990 Dec 1;50(23):7513–7518. [PubMed] [Google Scholar]
- Pera M. F., Friedlos F., Mills J., Roberts J. J. Inherent sensitivity of cultured human embryonal carcinoma cells to adducts of cis-diamminedichloroplatinum(II) on DNA. Cancer Res. 1987 Dec 15;47(24 Pt 1):6810–6813. [PubMed] [Google Scholar]
- Peters R. H., Jollow D. J., Stuart R. K. Role of glutathione in the in vitro synergism between 4-hydroperoxy-cyclophosphamide and cisplatin in leukemia cell lines. Cancer Res. 1991 May 15;51(10):2536–2541. [PubMed] [Google Scholar]
- Peters W. H., Boon C. E., Roelofs H. M., Wobbes T., Nagengast F. M., Kremers P. G. Expression of drug-metabolizing enzymes and P-170 glycoprotein in colorectal carcinoma and normal mucosa. Gastroenterology. 1992 Aug;103(2):448–455. doi: 10.1016/0016-5085(92)90833-k. [DOI] [PubMed] [Google Scholar]
- Peters W. H., Nagengast F. M., Wobbes T. Glutathione S-transferases in normal and cancerous human colon tissue. Carcinogenesis. 1989 Dec;10(12):2371–2374. doi: 10.1093/carcin/10.12.2371. [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Gopal V., Banavali S. D., Finke D., Bokari S. A. Multiparameter assessment of the cell cycle effects of bioactive and cytotoxic agents. Cancer Res. 1992 Aug 1;52(15):4090–4095. [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Strohmeyer T., Klöne A., Wagner G., Hartmann M., Sies H. Glutathione S-transferases in human testicular germ cell tumors: changes of expression and activity. J Urol. 1992 May;147(5):1424–1428. doi: 10.1016/s0022-5347(17)37586-9. [DOI] [PubMed] [Google Scholar]
- Timmer-Bosscha H., Hospers G. A., Meijer C., Mulder N. H., Muskiet F. A., Martini I. A., Uges D. R., de Vries E. G. Influence of docosahexaenoic acid on cisplatin resistance in a human small cell lung carcinoma cell line. J Natl Cancer Inst. 1989 Jul 19;81(14):1069–1075. doi: 10.1093/jnci/81.14.1069. [DOI] [PubMed] [Google Scholar]
- Timmer-Bosscha H., Timmer A., Meijer C., de Vries E. G., de Jong B., Oosterhuis J. W., Mulder N. H. cis-diamminedichloroplatinum(ii) resistance in vitro and in vivo in human embryonal carcinoma cells. Cancer Res. 1993 Dec 1;53(23):5707–5713. [PubMed] [Google Scholar]
- Waalkes M. P., Perantoni A. Isolation of a novel metal-binding protein from rat testes. Characterization and distinction from metallothionein. J Biol Chem. 1986 Oct 5;261(28):13097–13103. [PubMed] [Google Scholar]
- Walker M. C., Parris C. N., Masters J. R. Differential sensitivities of human testicular and bladder tumor cell lines to chemotherapeutic drugs. J Natl Cancer Inst. 1987 Aug;79(2):213–216. [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., Mulder N. H., de Vries E. G. Lack of cross-resistance to fostriecin in a human small-cell lung carcinoma cell line showing topoisomerase II-related drug resistance. Cancer Chemother Pharmacol. 1991;28(6):461–464. doi: 10.1007/BF00685823. [DOI] [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]


