Abstract
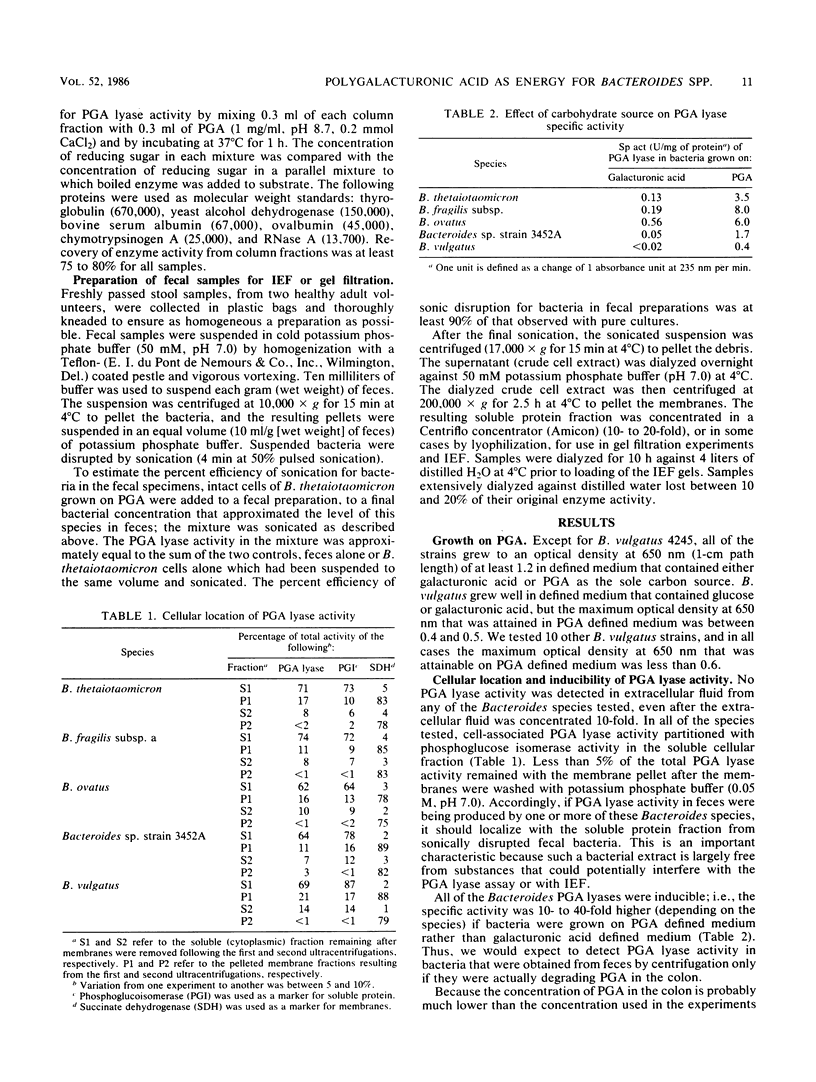
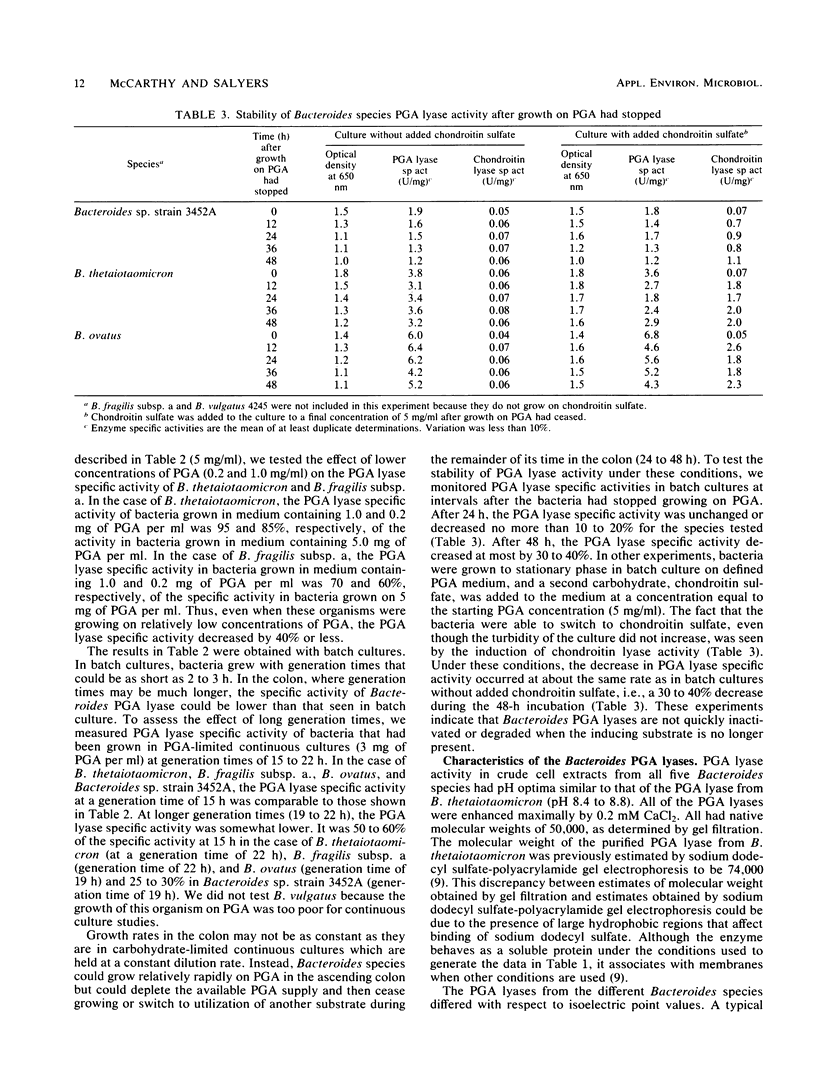
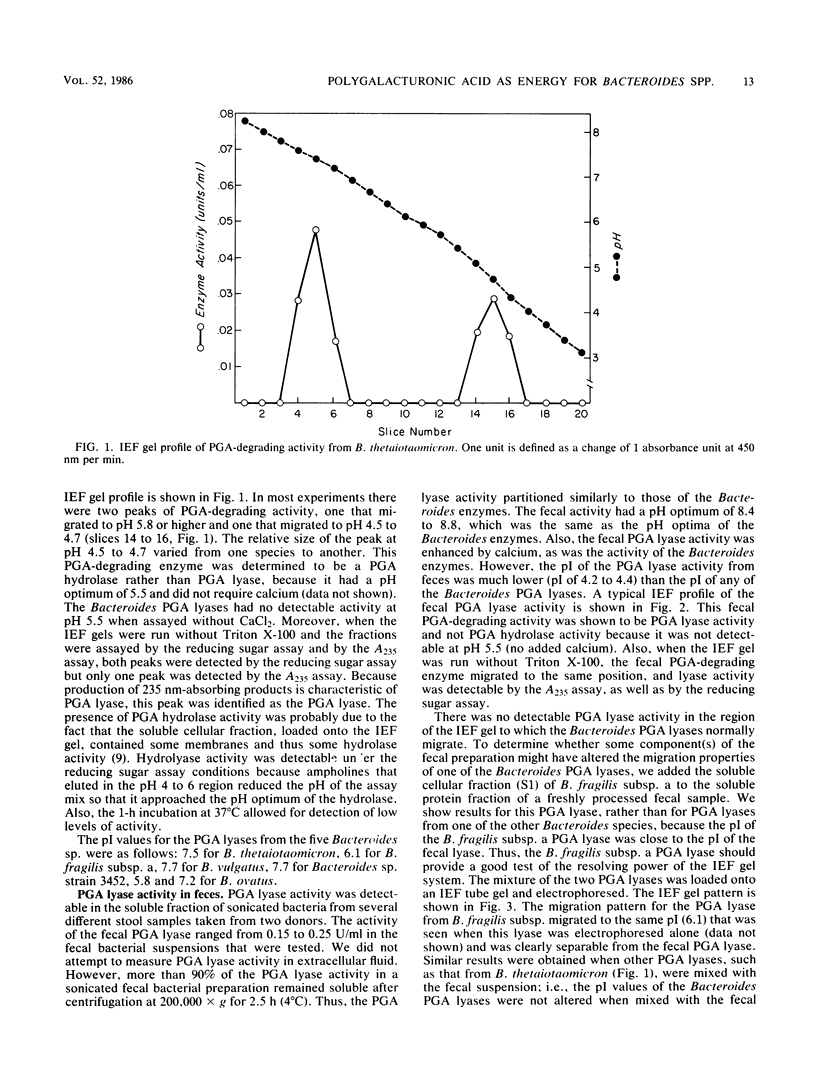
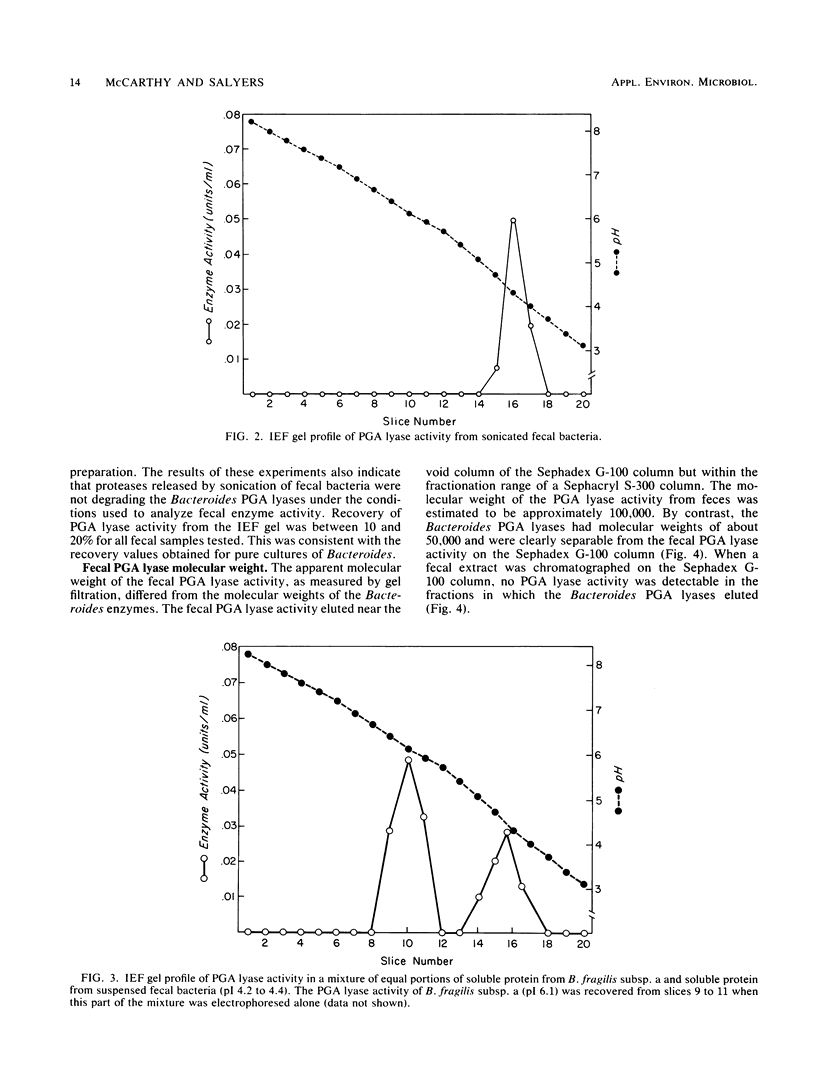
Five Bacteroides species that are found in the human colon can utilize polygalacturonic acid (PGA) when they are grown in laboratory media: Bacteroides thetaiotaomicron, Bacteroides vulgatus, Bacteroides ovatus, Bacteroides fragilis subsp. a, and Bacteroides sp. strain 3452A (an unnamed DNA-DNA homology group). PGA-degrading enzymes from B. thetaiotaomicron have been isolated and characterized previously. To determine whether a PGA lyase activity in human feces could be attributed to any of these species, we first determined the properties of PGA lyases from the other four Bacteroides species. PGA lyases from all the Bacteroides species were soluble, cell associated, and inducible by PGA. All had similar pH optima (8.4 to 8.8) and similar molecular weights (50,000). All activities were enhanced by calcium. The PGA lyases from the five species differed with respect to isoelectric point: B. thetaiotaomicron (pI 7.5), B. vulgatus (pI 7.7), B. ovatus (pI 5.8, 7.2), B. fragilis subsp. a (pI 6.1), and Bacteroides sp. strain 3452A (pI 7.7). The PGA lyase activity in human feces resembled those of the Bacteroides PGA lyases in that it had a pH optimum of 8.4 to 8.8 and was enhanced by calcium. However, it differed from the Bacteroides PGA lyases both with respect to isoelectric point (pI 4.2 to 4.4) and molecular weight (100,000). On the basis of these findings, it appears that the PGA lyase activity in human feces is not produced by any of the Bacteroides species surveyed in this survey. Moreover, there was no detectable PGA lyase activity in feces that had the same properties as the Bacteroides enzymes.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N. S., Canale-Parola E. Nutritionally limited pectinolytic bacteria from the human intestine. Appl Environ Microbiol. 1985 Jul;50(1):172–173. doi: 10.1128/aem.50.1.172-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Effect of long generation times on growth of Bacteroides thetaiotaomicron in carbohydrate-induced continuous culture. J Bacteriol. 1981 Jun;146(3):853–860. doi: 10.1128/jb.146.3.853-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritza A. P., Salyers A. A. Use of a species-specific DNA hybridization probe for enumerating Bacteroides vulgatus in human feces. Appl Environ Microbiol. 1985 Oct;50(4):958–964. doi: 10.1128/aem.50.4.958-964.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritza A. P., Shaughnessy P., Salyers A. A. Enumeration of polysaccharide-degrading Bacteroides species in human feces by using species-specific DNA probes. Appl Environ Microbiol. 1986 Feb;51(2):385–390. doi: 10.1128/aem.51.2.385-390.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarthy R. E., Kotarski S. F., Salyers A. A. Location and characteristics of enzymes involved in the breakdown of polygalacturonic acid by Bacteroides thetaiotaomicron. J Bacteriol. 1985 Feb;161(2):493–499. doi: 10.1128/jb.161.2.493-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Kotarski S. F. Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):781–788. doi: 10.1128/jb.143.2.781-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


