Abstract
The chemoprotective effect of vanadium, a dietary micronutrient, against chemically induced hepatocarcinogenesis in rats was investigated. Initiation was performed by a single intraperitoneal injection of diethylnitrosamine (DENA; 200 mg kg-1) followed by promotion with phenobarbital (0.05%) in the diet. Supplementary vanadium (0.5 p.p.m.) in the drinking water was provided ad libitum throughout the experiment, before the initiation or during the promotion period. At the end of the study (20 weeks), vanadium supplementation throughout the experiment reduced the incidence (P < 0.01), total number and multiplicity (P < 0.001) and altered the size distribution of visible persistent nodules (PNs) as compared with DENA control animals. Mean nodular volume (P < 0.05) and nodular volume as a percentage of liver volume (P < 0.01) were also attenuated following long-term vanadium treatment. It also caused a large decrease in the number (P < 0.001) and surface area (P < 0.01) of gamma-glutamyltranspeptidase (GGT)-positive hepatocyte foci and in the labelling index (P < 0.001) of focal cells, coupled with increased (P < 0.01) remodelling. The activity of GGT, measured quantitatively, was found to be significantly less in the PNs (P < 0.001) and non-nodular surrounding parenchyma (P < 0.01) of vanadium-supplemented rats. The anticarcinogenic effect of vanadium was also reflected in the histopathological analysis of liver sections that showed a well-maintained hepatocellular architecture as compared with DENA control. Similar results were observed when vanadium was given only before the initiation. However, supplementation of vanadium during the promotion period did not result in significant alterations of these parameters. Our results, thus, strongly suggest that vanadium may have a unique anti-tumour potential which is primarily exerted on the initiation phase and only secondarily on the promotion stage.
Full text
PDF
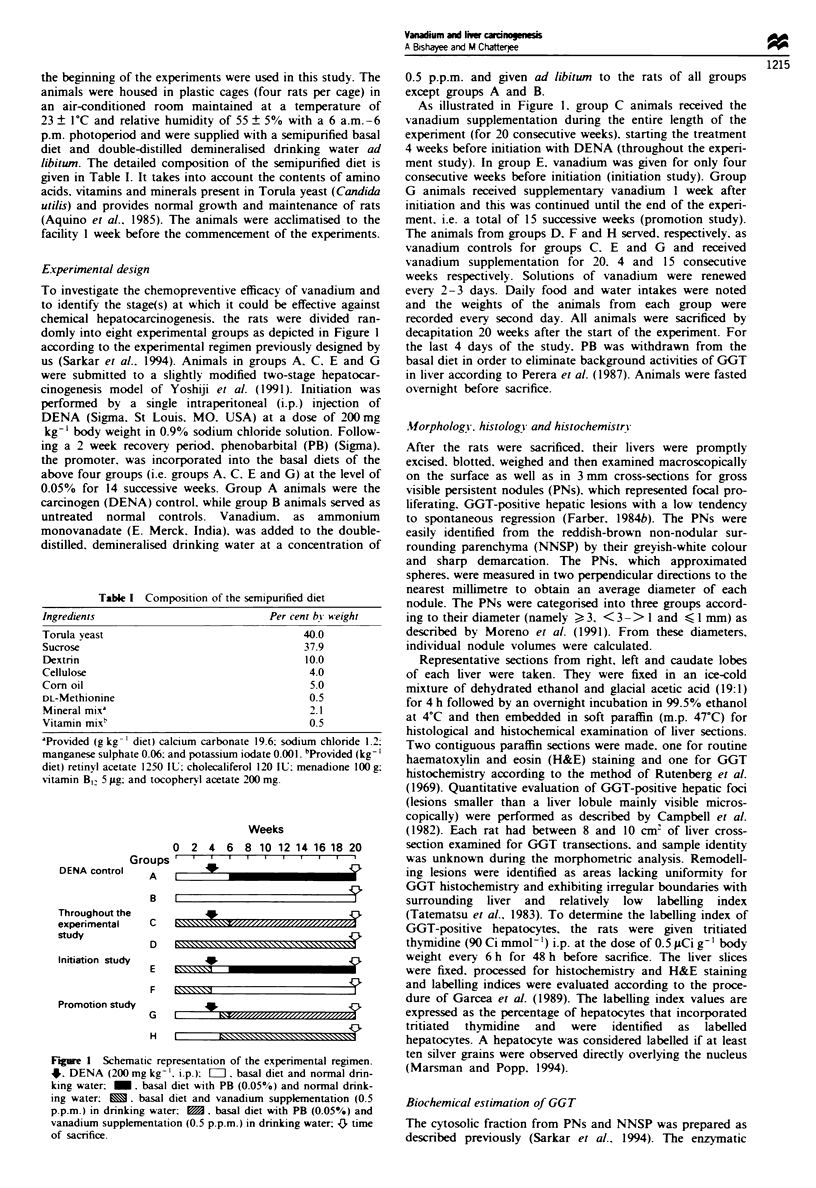
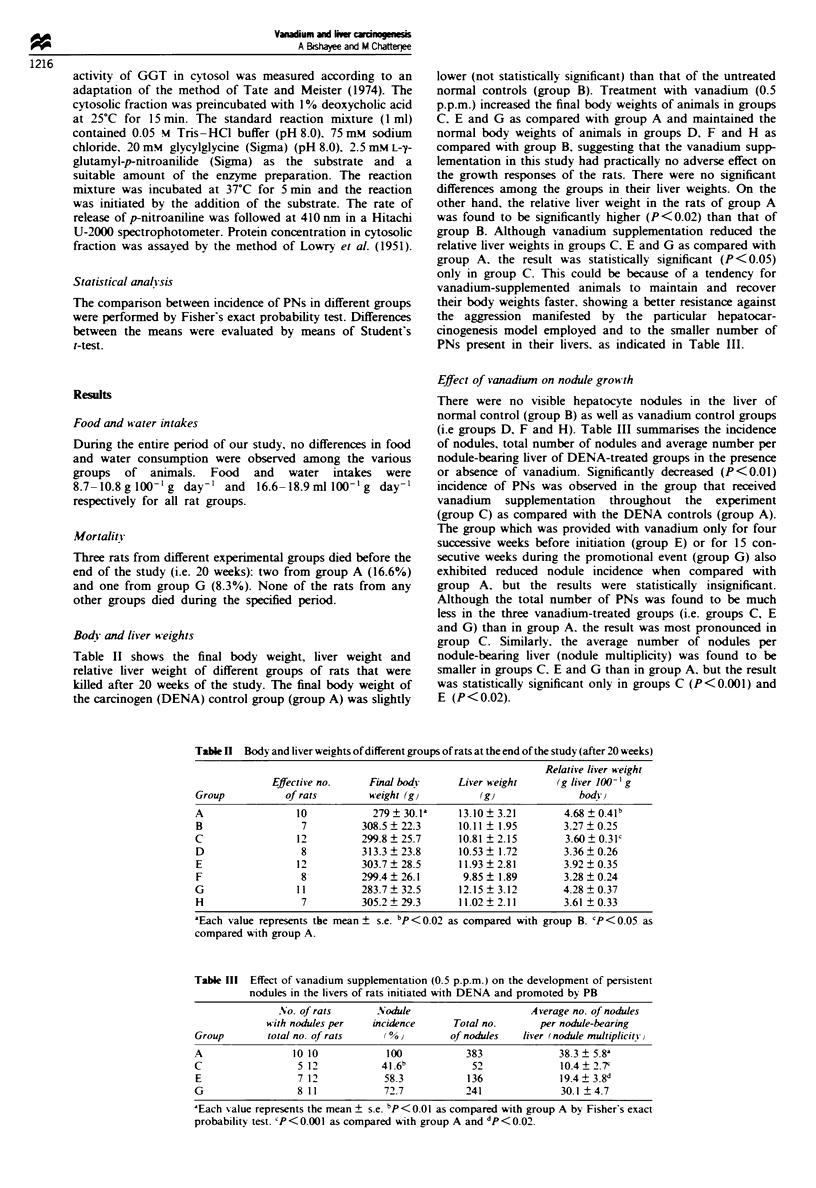




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishayee A., Chatterjee M. Selective enhancement of glutathione S-transferase activity in liver and extrahepatic tissues of rat following oral administration of vanadate. Acta Physiol Pharmacol Bulg. 1993;19(3):83–89. [PubMed] [Google Scholar]
- Cameron R., Kellen J., Kolin A., Malkin A., Farber E. Gamma-glutamyltransferase in putative premalignant liver cell populations during hepatocarcinogenesis. Cancer Res. 1978 Mar;38(3):823–829. [PubMed] [Google Scholar]
- Campbell H. A., Pitot H. C., Potter V. R., Laishes B. A. Application of quantitative stereology to the evaluation of enzyme-altered foci in rat liver. Cancer Res. 1982 Feb;42(2):465–472. [PubMed] [Google Scholar]
- Chakraborty A., Chatterjee M. Enhanced erythropoietin and suppression of gamma-glutamyl transpeptidase (GGT) activity in murine lymphoma following administration of vanadium. Neoplasma. 1994;41(5):291–296. [PubMed] [Google Scholar]
- Coker H. A., Thomas A. E., Akintonwa A. Determination of the total level of nitrosamines in select consumer products in Lagos area of Nigeria. Bull Environ Contam Toxicol. 1991 Nov;47(5):706–710. doi: 10.1007/BF01701138. [DOI] [PubMed] [Google Scholar]
- Djordjevic C., Wampler G. L. Antitumor activity and toxicity of peroxo heteroligand vanadates(V) in relation to biochemistry of vanadium. J Inorg Biochem. 1985 Sep;25(1):51–55. doi: 10.1016/0162-0134(85)83007-5. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Farber E. Cellular biochemistry of the stepwise development of cancer with chemicals: G. H. A. Clowes memorial lecture. Cancer Res. 1984 Dec;44(12 Pt 1):5463–5474. [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem Pharmacol. 1990 Jun 15;39(12):1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Farber E. The sequential analysis of liver cancer induction. Biochim Biophys Acta. 1980 May 6;605(2):149–166. doi: 10.1016/0304-419x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Fiala S., Fiala E. S. Activation by chemical carcinogens of gamma-glutamyl transpeptidase in rat and mouse liver. J Natl Cancer Inst. 1973 Jul;51(1):151–158. doi: 10.1093/jnci/51.1.151. [DOI] [PubMed] [Google Scholar]
- French R. J., Jones P. J. Role of vanadium in nutrition: metabolism, essentiality and dietary considerations. Life Sci. 1993;52(4):339–346. doi: 10.1016/0024-3205(93)90146-t. [DOI] [PubMed] [Google Scholar]
- Garcea R., Daino L., Pascale R., Simile M. M., Puddu M., Frassetto S., Cozzolino P., Seddaiu M. A., Gaspa L., Feo F. Inhibition of promotion and persistent nodule growth by S-adenosyl-L-methionine in rat liver carcinogenesis: role of remodeling and apoptosis. Cancer Res. 1989 Apr 1;49(7):1850–1856. [PubMed] [Google Scholar]
- Greenwald P., Cullen J. W., McKenna J. W. Cancer prevention and control: from research through applications. J Natl Cancer Inst. 1987 Aug;79(2):389–400. [PubMed] [Google Scholar]
- Gullapalli S., Shivaswamy V., Ramasarma T., Kurup C. K. Redistribution of subcellular calcium in rat liver on administration of vanadate. Mol Cell Biochem. 1989 Oct 31;90(2):155–164. doi: 10.1007/BF00221215. [DOI] [PubMed] [Google Scholar]
- Hanauske U., Hanauske A. R., Marshall M. H., Muggia V. A., Von Hoff D. D. Biphasic effect of vanadium salts on in vitro tumor colony growth. Int J Cell Cloning. 1987 Mar;5(2):170–178. doi: 10.1002/stem.5530050209. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Pitot H. C. Gamma-glutamyl transpeptidase--its role in hepatocarcinogenesis. Carcinogenesis. 1985 Feb;6(2):165–172. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- Kingsnorth A. N., LaMuraglia G. M., Ross J. S., Malt R. A. Vanadate supplements and 1,2-dimethylhydrazine induced colon cancer in mice: increased thymidine incorporation without enhanced carcinogenesis. Br J Cancer. 1986 May;53(5):683–686. doi: 10.1038/bjc.1986.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpf-Maier P. Cytostatische Nicht-Platinmetall-Komplexe: neue Perspektiven für die Krebsbehandlung? Naturwissenschaften. 1987 Aug;74(8):374–382. doi: 10.1007/BF00405465. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsman D. S., Popp J. A. Biological potential of basophilic hepatocellular foci and hepatic adenoma induced by the peroxisome proliferator, Wy-14,643. Carcinogenesis. 1994 Jan;15(1):111–117. doi: 10.1093/carcin/15.1.111. [DOI] [PubMed] [Google Scholar]
- Moreno F. S., Rizzi M. B., Dagli M. L., Penteado M. V. Inhibitory effects of beta-carotene on preneoplastic lesions induced in Wistar rats by the resistant hepatocyte model. Carcinogenesis. 1991 Oct;12(10):1817–1822. doi: 10.1093/carcin/12.10.1817. [DOI] [PubMed] [Google Scholar]
- Ould Elhkim M., Decloître F., Martin M., Loridon-Rosa B., Frayssinet C. Role of diethylnitrosamine, 2-acetylaminofluorene and partial hepatectomy in the expression of glutathione-S-transferase-P and gamma-glutamyltranspeptidase in the early steps of rat liver carcinogenesis. Tumour Biol. 1992;13(3):152–161. doi: 10.1159/000217759. [DOI] [PubMed] [Google Scholar]
- Perera M. I., Katyal S. L., Shinozuka H. Choline deficient diet enhances the initiating and promoting effects of methapyrilene hydrochloride in rat liver as assayed by the induction of gamma-glutamyltranspeptidase-positive hepatocyte foci. Br J Cancer. 1987 Dec;56(6):774–778. doi: 10.1038/bjc.1987.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C., Campbell H. A., Maronpot R., Bawa N., Rizvi T. A., Xu Y. H., Sargent L., Dragan Y., Pyron M. Critical parameters in the quantitation of the stages of initiation, promotion, and progression in one model of hepatocarcinogenesis in the rat. Toxicol Pathol. 1989;17(4 Pt 1):594–612. doi: 10.1177/0192623389017004105. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sabbioni E., Pozzi G., Devos S., Pintar A., Casella L., Fischbach M. The intensity of vanadium(V)-induced cytotoxicity and morphological transformation in BALB/3T3 cells is dependent on glutathione-mediated bioreduction to vanadium(IV). Carcinogenesis. 1993 Dec;14(12):2565–2568. doi: 10.1093/carcin/14.12.2565. [DOI] [PubMed] [Google Scholar]
- Sabbioni E., Pozzi G., Pintar A., Casella L., Garattini S. Cellular retention, cytotoxicity and morphological transformation by vanadium(IV) and vanadium(V) in BALB/3T3 cell lines. Carcinogenesis. 1991 Jan;12(1):47–52. doi: 10.1093/carcin/12.1.47. [DOI] [PubMed] [Google Scholar]
- Sardar S., Ghosh R., Mondal A., Chatterjee M. Protective role of vanadium in the survival of hosts during the growth of a transplantable murine lymphoma and its profound effects on the rates and patterns of biotransformation. Neoplasma. 1993;40(1):27–30. [PubMed] [Google Scholar]
- Sarkar A., Mukherjee B., Chatterjee M. Inhibitory effect of beta-carotene on chronic 2-acetylaminofluorene induced hepatocarcinogenesis in rat: reflection in hepatic drug metabolism. Carcinogenesis. 1994 May;15(5):1055–1060. doi: 10.1093/carcin/15.5.1055. [DOI] [PubMed] [Google Scholar]
- Scherer E., Emmelot P. Kinetics of induction and growth of enzyme-deficient islands involved in hepatocarcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2544–2554. [PubMed] [Google Scholar]
- Serfontein W. J., Hurter P. Nitrosamines as environmental carcinogens. II. Evidence for the presence of nitrosamines in tobacco smoke condensate. Cancer Res. 1966 Apr;26(4):575–579. [PubMed] [Google Scholar]
- Shirai T., Imaida K., Ohshima M., Fukushima S., Lee M. S., King C. M., Ito N. Different responses to phenobarbital promotion in the development of gamma-glutamyltranspeptidase-positive foci in the liver of rats initiated with diethylnitrosamine, N-hydroxy-2-acetyl-aminofluorene and aflatoxin B1. Jpn J Cancer Res. 1985 Jan;76(1):16–19. [PubMed] [Google Scholar]
- Swenberg J. A., Hoel D. G., Magee P. N. Mechanistic and statistical insight into the large carcinogenesis bioassays on N-nitrosodiethylamine and N-nitrosodimethylamine. Cancer Res. 1991 Dec 1;51(23 Pt 2):6409–6414. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Tatematsu M., Mera Y., Inoue T., Satoh K., Sato K., Ito N. Stable phenotypic expression of glutathione S-transferase placental type and unstable phenotypic expression of gamma-glutamyltransferase in rat liver preneoplastic and neoplastic lesions. Carcinogenesis. 1988 Feb;9(2):215–220. doi: 10.1093/carcin/9.2.215. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Nagamine Y., Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983 Nov;43(11):5049–5058. [PubMed] [Google Scholar]
- Thompson H. J., Chasteen N. D., Meeker L. D. Dietary vanadyl(IV) sulfate inhibits chemically-induced mammary carcinogenesis. Carcinogenesis. 1984 Jun;5(6):849–851. doi: 10.1093/carcin/5.6.849. [DOI] [PubMed] [Google Scholar]
- Waitzberg D. L., Gonçalves E. L., Faintuch J., Bevilacqua L. R., Rocha C. L., Cologni A. M. Effect of diets with different protein levels on the growth of Walker 256 carcinosarcoma in rats. Braz J Med Biol Res. 1989;22(4):447–455. [PubMed] [Google Scholar]
- Williams G. M. The pathogenesis of rat liver cancer caused by chemical carcinogens. Biochim Biophys Acta. 1980 May 6;605(2):167–189. doi: 10.1016/0304-419x(80)90003-7. [DOI] [PubMed] [Google Scholar]
- Williams G. M. The significance of chemically-induced hepatocellular altered foci in rat liver and application to carcinogen detection. Toxicol Pathol. 1989;17(4 Pt 1):663–674. doi: 10.1177/0192623389017004111. [DOI] [PubMed] [Google Scholar]
- Yoshiji H., Nakae D., Kinugasa T., Matsuzaki M., Denda A., Tsujii T., Konishi Y. Inhibitory effect of dietary iron deficiency on the induction of putative preneoplastic foci in rat liver initiated with diethylnitrosamine and promoted by phenobarbital. Br J Cancer. 1991 Nov;64(5):839–842. doi: 10.1038/bjc.1991.410. [DOI] [PMC free article] [PubMed] [Google Scholar]


