Abstract
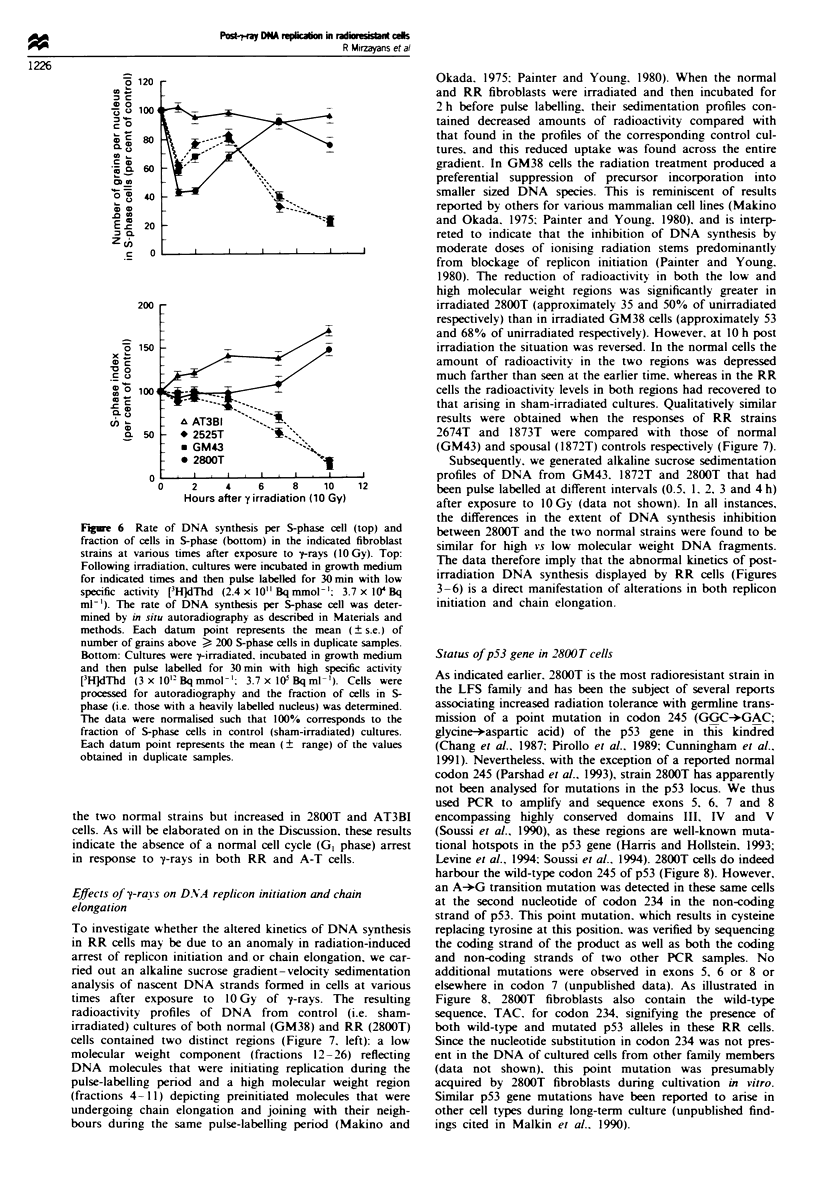
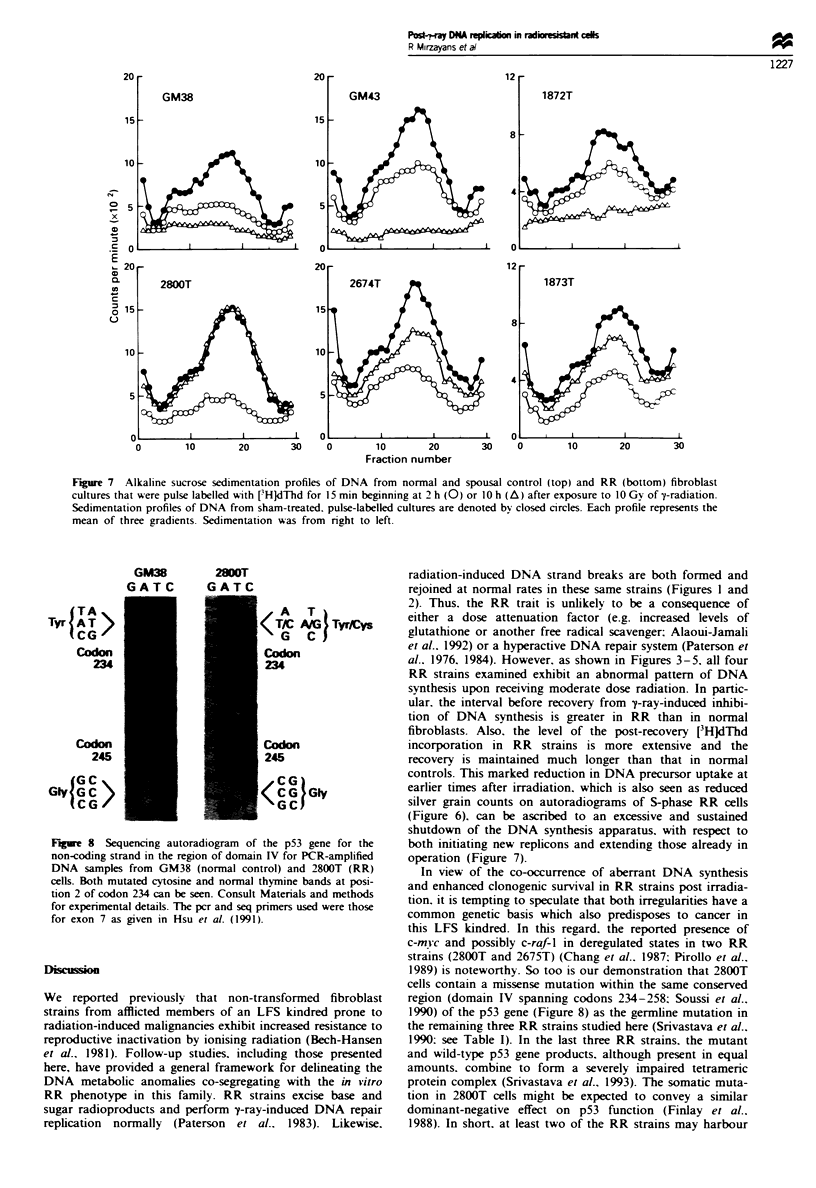
Non-malignant dermal fibroblast strains, cultured from affected members of a Li-Fraumeni syndrome (LFS) family with diverse neoplasms associated with radiation exposure, display a unique increased resistance to the lethal effects of gamma-radiation. In the studies reported here, this radioresistance (RR) trait has been found to correlate strongly with an abnormal pattern of post-gamma-ray DNA replicative synthesis, as monitored by radiolabelled thymidine incorporation and S-phase cell autoradiography. In particular, the time interval between the gamma-ray-induced shutdown of DNA synthesis and its subsequent recovery was greater in all four RR strains examined and the post-recovery replication rate was much higher and was maintained longer than in normal and spousal controls. Alkaline sucrose sedimentation profiles of pulse-labelled cellular DNA indicated that the unusual pattern of DNA replication in irradiated RR strains may be ascribed to anomalies in both replicon initiation and DNA chain elongation processes. Moreover, the RR strain which had previously displayed the highest post-gamma-ray clonogenic survival was found to harbour a somatic (codon 234) mutation (presumably acquired during culture in vitro) in the same conserved region of the p53 tumour-suppressor gene as the germline (codon 245) mutation in the remaining three RR strains from other family members, thus coupling the RR phenotype and abnormal post-gamma-ray DNA synthesis pattern with faulty p53 expression. Significantly, these two aberrant radioresponse end points, along with documented anomalies in c-myc and c-raf-1 proto-oncogenes, are unprecedented among other LFS families carrying p53 germline mutations. We thus speculate that this peculiar cancer-prone family may possess in its germ line a second, as yet unidentified, genetic defect in addition to the p53 mutation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
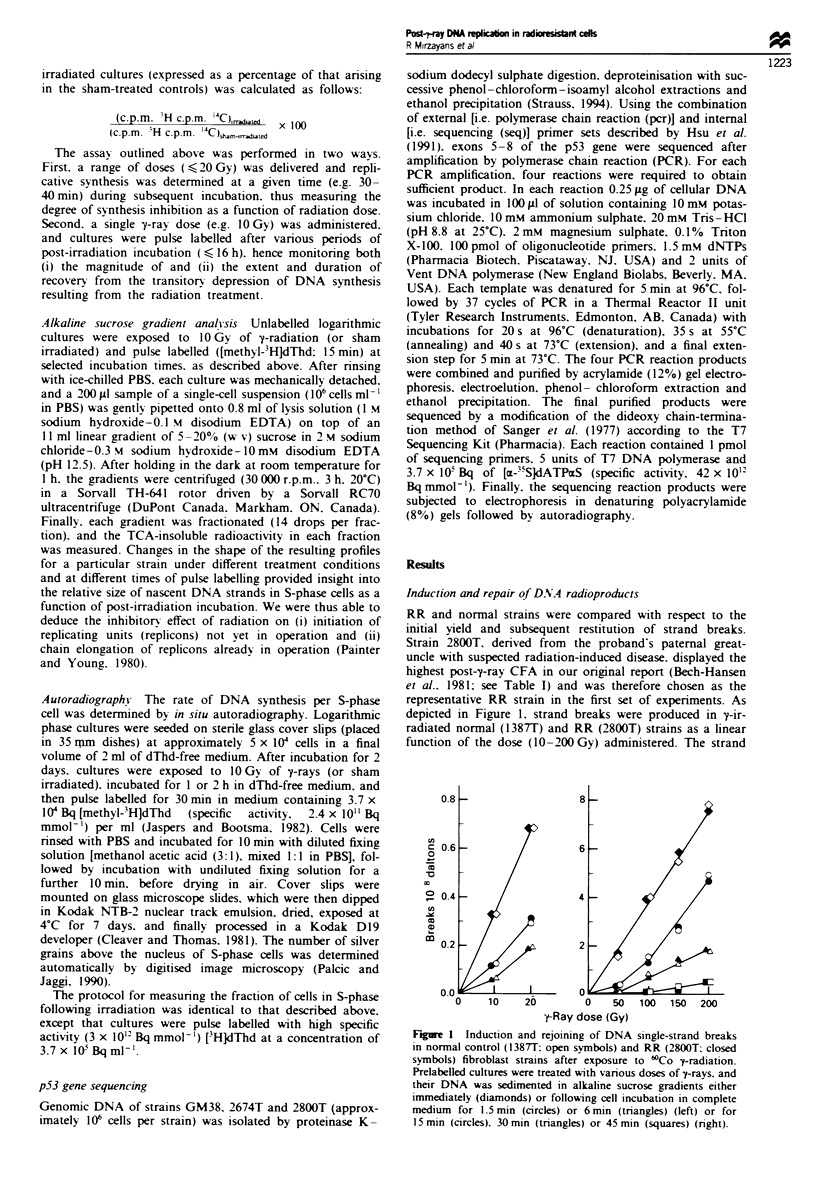
- Alaoui-Jamali M. A., Batist G., Lehnert S. Radiation-induced damage to DNA in drug- and radiation-resistant sublines of a human breast cancer cell line. Radiat Res. 1992 Jan;129(1):37–42. [PubMed] [Google Scholar]
- Bech-Hansen N. T., Blattner W. A., Sell B. M., McKeen E. A., Lampkin B. C., Fraumeni J. F., Jr, Paterson M. C. Transmission of in-vitro radioresistance in a cancer-prone family. Lancet. 1981 Jun 20;1(8234):1335–1337. doi: 10.1016/s0140-6736(81)92517-4. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., McGuire D. B., Mulvihill J. J., Lampkin B. C., Hananian J., Fraumeni J. F. Genealogy of cancer in a family. JAMA. 1979 Jan 19;241(3):259–261. [PubMed] [Google Scholar]
- Chang E. H., Pirollo K. F., Zou Z. Q., Cheung H. Y., Lawler E. L., Garner R., White E., Bernstein W. B., Fraumeni J. W., Jr, Blattner W. A. Oncogenes in radioresistant, noncancerous skin fibroblasts from a cancer-prone family. Science. 1987 Aug 28;237(4818):1036–1039. doi: 10.1126/science.3616624. [DOI] [PubMed] [Google Scholar]
- Cunningham J. M., Francis G. E., Holland M. J., Pirollo K. F., Chang E. H. Aberrant DNA topoisomerase II activity, radioresistance and inherited susceptibility to cancer. Br J Cancer. 1991 Jan;63(1):29–36. doi: 10.1038/bjc.1991.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
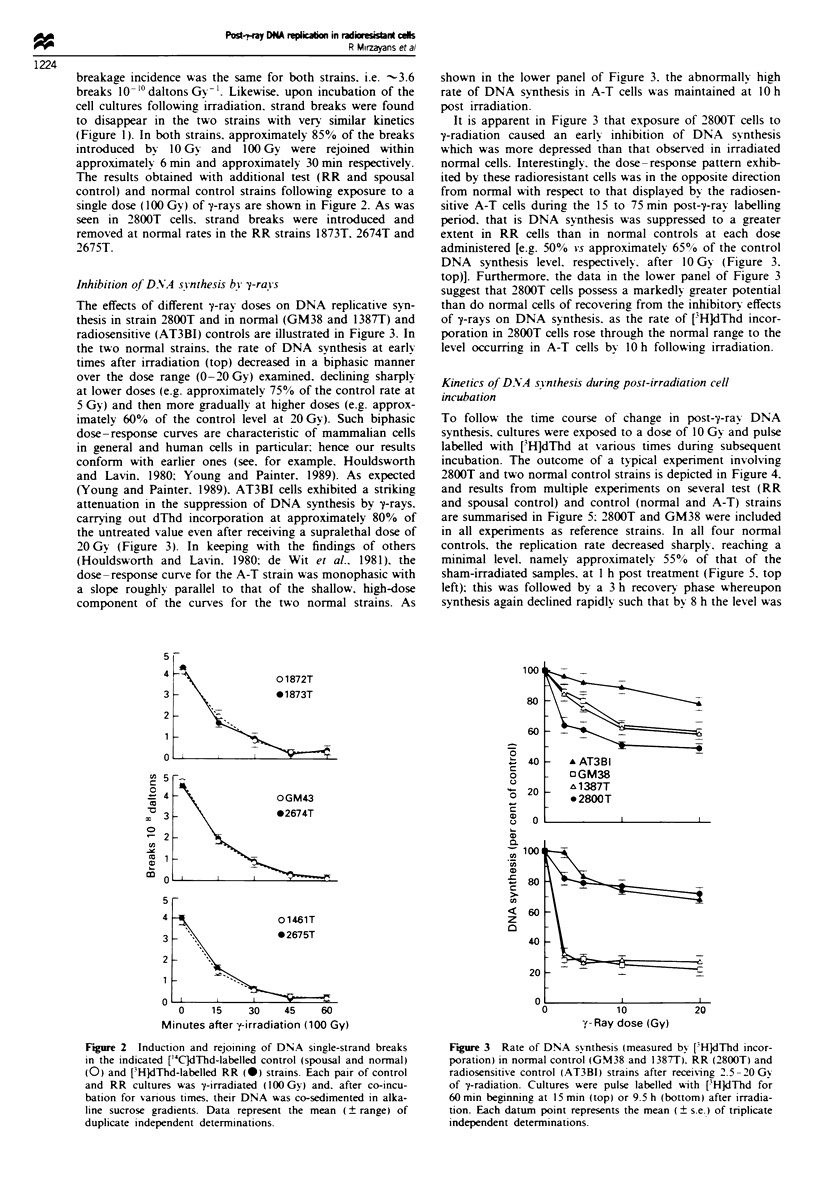
- Houldsworth J., Lavin M. F. Effect of ionizing radiation on DNA synthesis in ataxia telangiectasia cells. Nucleic Acids Res. 1980 Aug 25;8(16):3709–3720. doi: 10.1093/nar/8.16.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Jaspers N. G., Bootsma D. Genetic heterogeneity in ataxia-telangiectasia studied by cell fusion. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2641–2644. doi: 10.1073/pnas.79.8.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
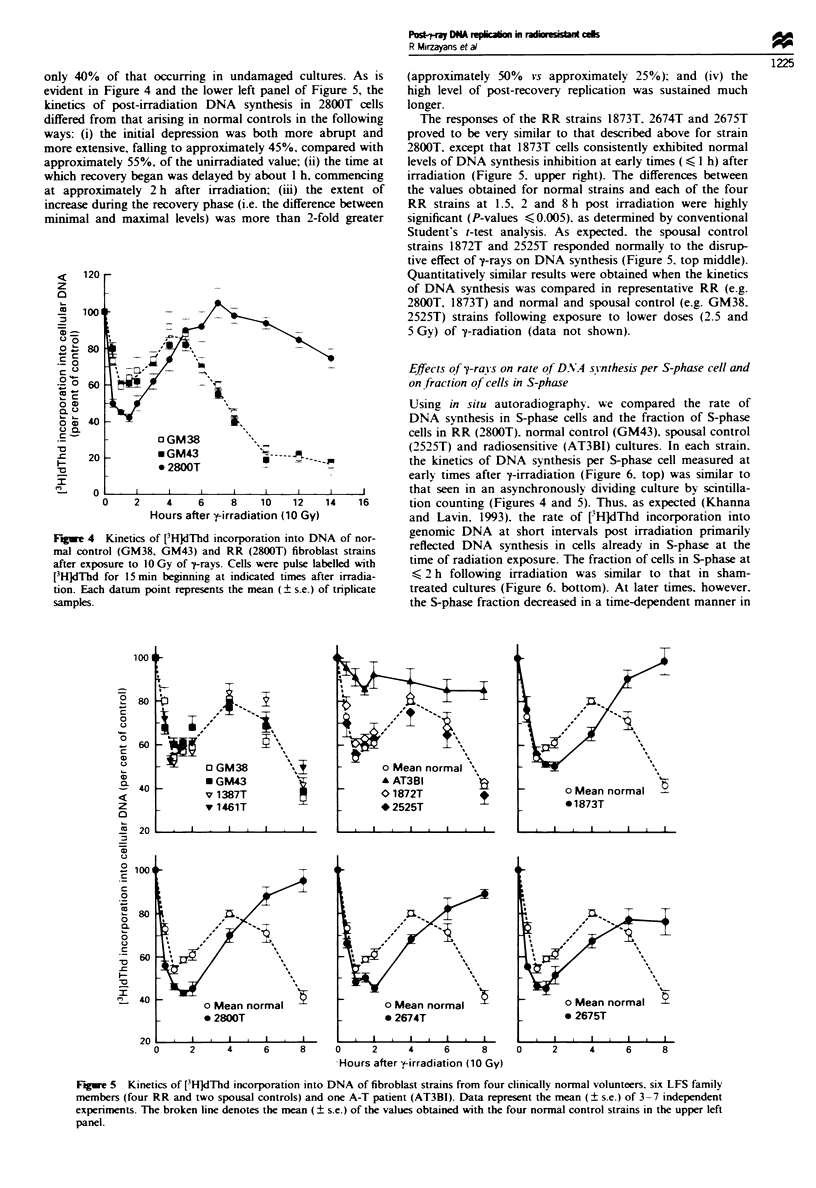
- Jaspers N. G., Gatti R. A., Baan C., Linssen P. C., Bootsma D. Genetic complementation analysis of ataxia telangiectasia and Nijmegen breakage syndrome: a survey of 50 patients. Cytogenet Cell Genet. 1988;49(4):259–263. doi: 10.1159/000132673. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Khanna K. K., Lavin M. F. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene. 1993 Dec;8(12):3307–3312. [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Perry M. E., Chang A., Silver A., Dittmer D., Wu M., Welsh D. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer. 1994 Mar;69(3):409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Little J. B., Nove J., Dahlberg W. K., Troilo P., Nichols W. W., Strong L. C. Normal cytotoxic response of skin fibroblasts from patients with Li-Fraumeni familial cancer syndrome to DNA-damaging agents in vitro. Cancer Res. 1987 Aug 1;47(15):4229–4234. [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Magnuson N. S., Beck T., Vahidi H., Hahn H., Smola U., Rapp U. R. The Raf-1 serine/threonine protein kinase. Semin Cancer Biol. 1994 Aug;5(4):247–253. [PubMed] [Google Scholar]
- Makino F., Okada S. Effects of ionizing radiation on DNA replication in cultured mammalian cells. Radiat Res. 1975 Apr;62(1):37–51. [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Mirzayans R., Waters R., Paterson M. C. Induction and repair of DNA strand breaks and 1-beta-D-arabinofuranosylcytosine-detectable sites in 40-75 kVp X-irradiated compared to 60Co gamma-irradiated human cell lines. Radiat Res. 1988 Apr;114(1):168–185. [PubMed] [Google Scholar]
- Murnane J. P., Kapp L. N. A critical look at the association of human genetic syndromes with sensitivity to ionizing radiation. Semin Cancer Biol. 1993 Apr;4(2):93–104. [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshad R., Price F. M., Pirollo K. F., Chang E. H., Sanford K. K. Cytogenetic response to G2-phase X irradiation in relation to DNA repair and radiosensitivity in a cancer-prone family with Li-Fraumeni syndrome. Radiat Res. 1993 Nov;136(2):236–240. [PubMed] [Google Scholar]
- Paterson M. C., Gentner N. E., Middlestadt M. V., Weinfeld M. Cancer predisposition, carcinogen hypersensitivity, and aberrant DNA metabolism. J Cell Physiol Suppl. 1984;3:45–62. doi: 10.1002/jcp.1041210408. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Sell B. M., Smith B. P., Bech-Hansen N. T. Impaired colony-forming ability following gamma irradiation of skin fibroblasts from tuberous sclerosis patients. Radiat Res. 1982 May;90(2):260–270. [PubMed] [Google Scholar]
- Paterson M. C., Smith B. P., Lohman P. H., Anderson A. K., Fishman L. Defective excision repair of gamma-ray-damaged DNA in human (ataxia telangiectasia) fibroblasts. Nature. 1976 Apr 1;260(5550):444–447. doi: 10.1038/260444a0. [DOI] [PubMed] [Google Scholar]
- Pirollo K. F., Garner R., Yuan S. Y., Li L., Blattner W. A., Chang E. H. raf involvement in the simultaneous genetic transfer of the radioresistant and transforming phenotypes. Int J Radiat Biol. 1989 May;55(5):783–796. doi: 10.1080/09553008914550831. [DOI] [PubMed] [Google Scholar]
- Prokocimer M., Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994 Oct 15;84(8):2391–2411. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Slichenmyer W. J., Nelson W. G., Slebos R. J., Kastan M. B. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993 Sep 15;53(18):4164–4168. [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Wang S., Tong Y. A., Hao Z. M., Chang E. H. Dominant negative effect of a germ-line mutant p53: a step fostering tumorigenesis. Cancer Res. 1993 Oct 1;53(19):4452–4455. [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Stewart B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994 Sep 7;86(17):1286–1296. doi: 10.1093/jnci/86.17.1286. [DOI] [PubMed] [Google Scholar]
- Warenius H. M., Browning P. G., Britten R. A., Peacock J. A., Rapp U. R. C-raf-1 proto-oncogene expression relates to radiosensitivity rather than radioresistance. Eur J Cancer. 1994;30A(3):369–375. doi: 10.1016/0959-8049(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Young B. R., Painter R. B. Radioresistant DNA synthesis and human genetic diseases. Hum Genet. 1989 May;82(2):113–117. doi: 10.1007/BF00284040. [DOI] [PubMed] [Google Scholar]
- de Wit J., Jaspers N. G., Bootsma D. The rate of DNA synthesis in normal human and ataxia telangiectasia cells after exposure to X-irradiation. Mutat Res. 1981 Jan;80(1):221–226. doi: 10.1016/0027-5107(81)90190-1. [DOI] [PubMed] [Google Scholar]