Abstract
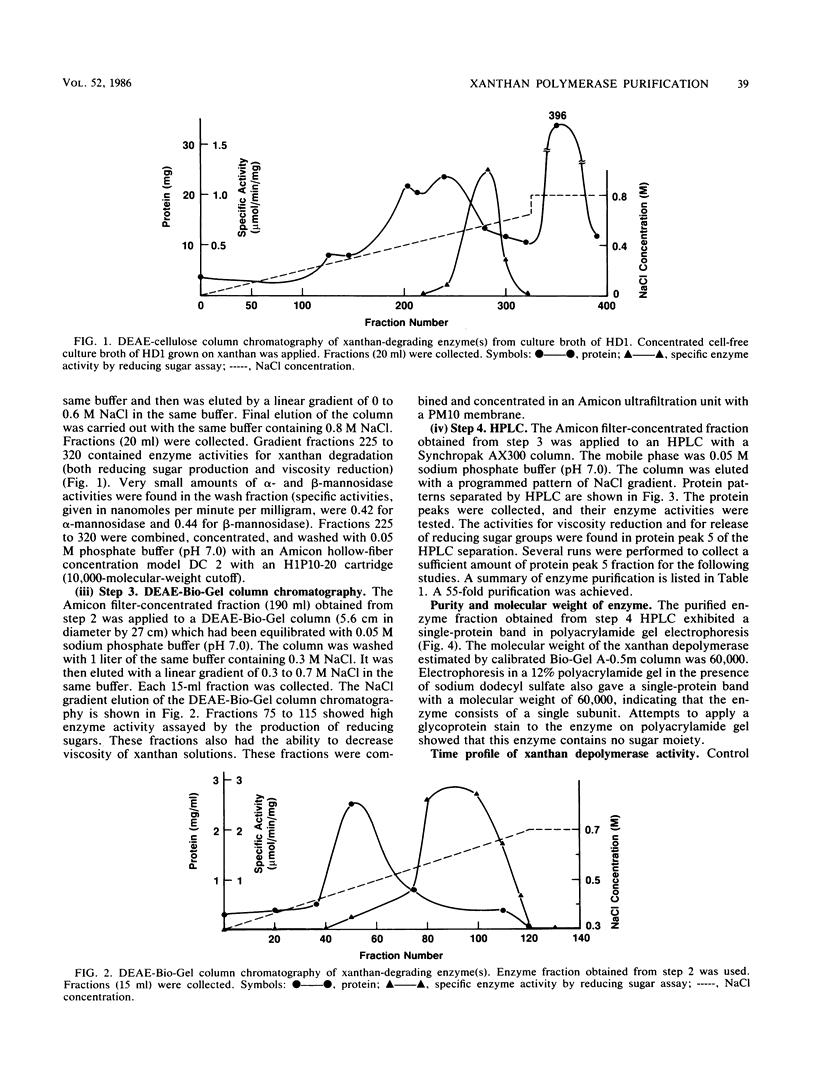
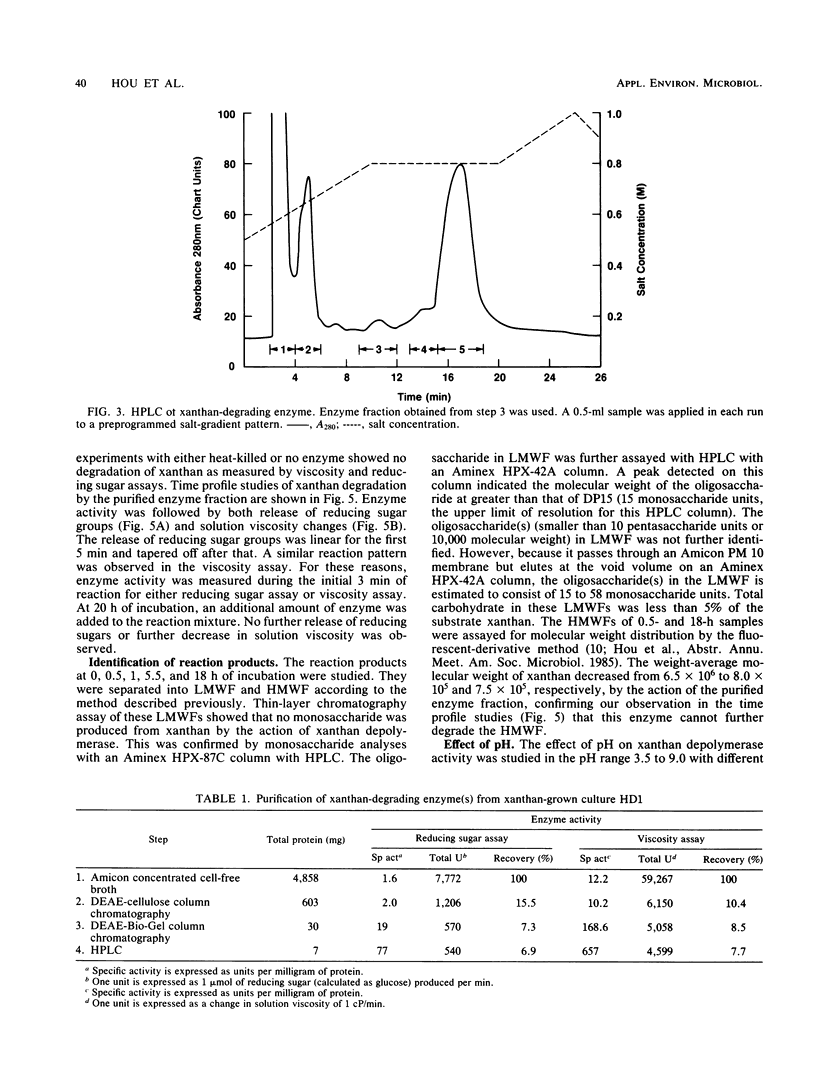
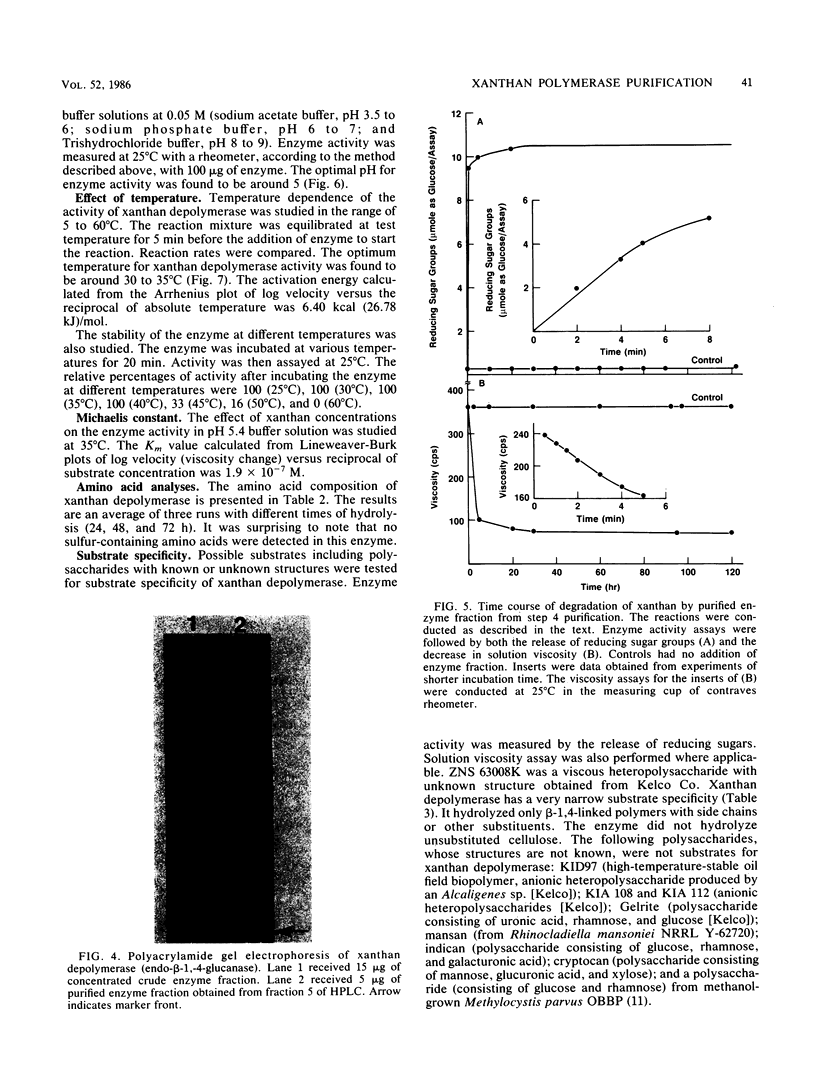
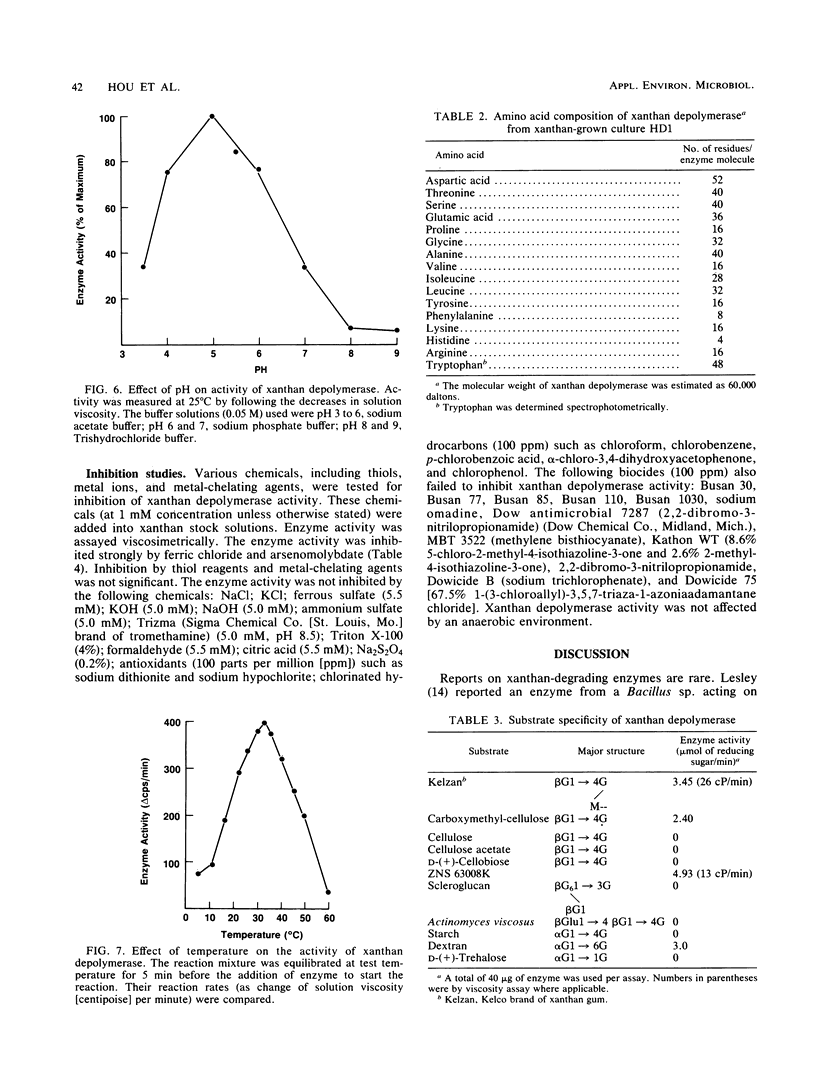
A novel xanthan depolymerase (endo-β-1,4-glucanase) was isolated from a salt-tolerant bacteria culture (HD1) grown on xanthan. The depolymerase was purified 55-fold through chromatography on ion-exchange and molecular sieve columns, including high-performance liquid chromatography. The purified enzyme fraction was homogeneous as judged by polyacrylamide gel electrophoresis. The molecular weight of this enzyme is 60,000. Optimum pH and temperature for xanthan depolymerase activity were around 5 and 30 to 35°C, respectively. The enzyme was not stable at a temperature higher than 45°C. The activation energy calculated from an Arrhenius plot was 6.40 kcal (26.78 kJ). The enzyme molecule contains no sugar moiety. The amino acid composition of the enzyme protein was determined. Xanthan depolymerase cleaves the endo-β-1,4-glucosidic linkage of the xanthan molecule, freeing reducing groups of some sugars and decreasing viscosity of the polymer solution. Only the backbones of β-1,4-linked glucans with side chains or other substituents were cleaved. No monosaccharide was produced by the action of this enzyme. The oligosac-charide(s) in the low-molecular weight fraction consisted of 15 to 58 monosaccharide units. The enzymic reaction resulted in the decrease in weight-average molecular weight of xanthan from 6.5 × 106 to 8.0 × 105 in 0.5 h. This enzyme alone could not degrade xanthan to a single or multiple pentasaccharide unit(s). Results suggest that there may be regions inside the xanthan molecule that are susceptible to the attack of this enzyme. Xanthan depolymerase activity was not inhibited by many chemicals, including thiols, antioxidants, chlorinated hydrocarbons, metal-chelating agents, and inorganic compounds, except ferric chloride and arsenomolybdate. Many biocides were tested and found not to be inhibitory. Conditions used in enhanced oil recovery operations, i.e., the presence of formaldehyde, Na2S2O4, 2,2-dibromo-3-nitrilopropionamide, and an anaerobic environment, did not inhibit xanthan depolymerase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beldman G., Searle-Van Leeuwen M. F., Rombouts F. M., Voragen F. G. The cellulase of Trichoderma viride. Purification, characterization and comparison of all detectable endoglucanases, exoglucanases and beta-glucosidases. Eur J Biochem. 1985 Jan 15;146(2):301–308. doi: 10.1111/j.1432-1033.1985.tb08653.x. [DOI] [PubMed] [Google Scholar]
- Bredderman P. J. Tryptophan analysis of proteins in 6M guanidine hydrochloride: modification for more general application. Anal Biochem. 1974 Sep;61(1):298–301. doi: 10.1016/0003-2697(74)90360-1. [DOI] [PubMed] [Google Scholar]
- Cadmus M. C., Jackson L. K., Burton K. A., Plattner R. D., Slodki M. E. Biodegradation of Xanthan Gum by Bacillus sp. Appl Environ Microbiol. 1982 Jul;44(1):5–11. doi: 10.1128/aem.44.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Griffin M. Affinity chromatography of the cellulase system of Trichoderma koningii. Biochem J. 1978 Mar 1;169(3):713–715. doi: 10.1042/bj1690713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Laskin A. I., Patel R. N. Growth and Polysaccharide Production by Methylocystis parvus OBBP on Methanol. Appl Environ Microbiol. 1979 May;37(5):800–804. doi: 10.1128/aem.37.5.800-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B. Structure of extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975 Dec;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- LESLEY S. M. Degradation of the polysaccharide of Xanthomonas phaseoli by an extracellular bacterial enzyme. Can J Microbiol. 1961 Oct;7:815–825. doi: 10.1139/m61-097. [DOI] [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- SCHWEIGER A. [Separation of simple sugars on cellulose lavers]. J Chromatogr. 1962 Nov;9:374–376. doi: 10.1016/s0021-9673(00)80803-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- de Belder A. N., Wik K. O. Preparation and properties of fluorescein-labelled hyaluronate. Carbohydr Res. 1975 Nov;44(2):251–257. doi: 10.1016/s0008-6215(00)84168-3. [DOI] [PubMed] [Google Scholar]



