Abstract
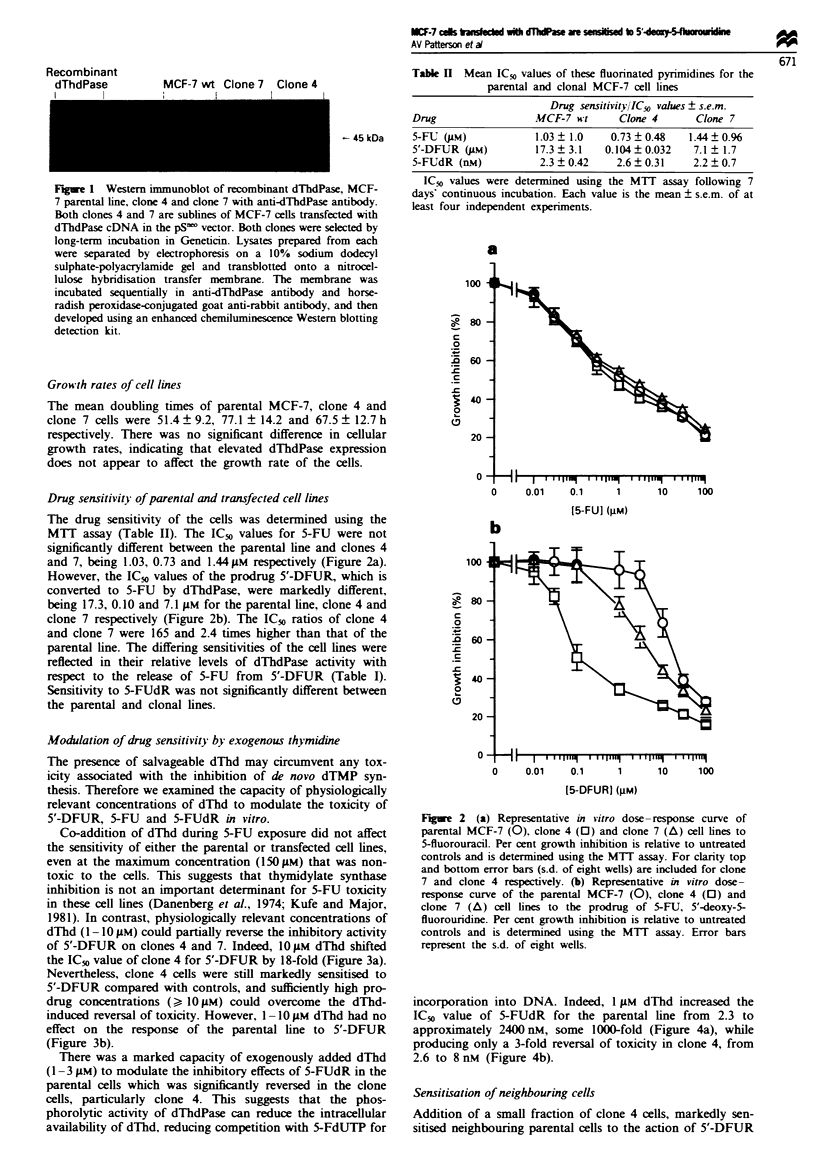
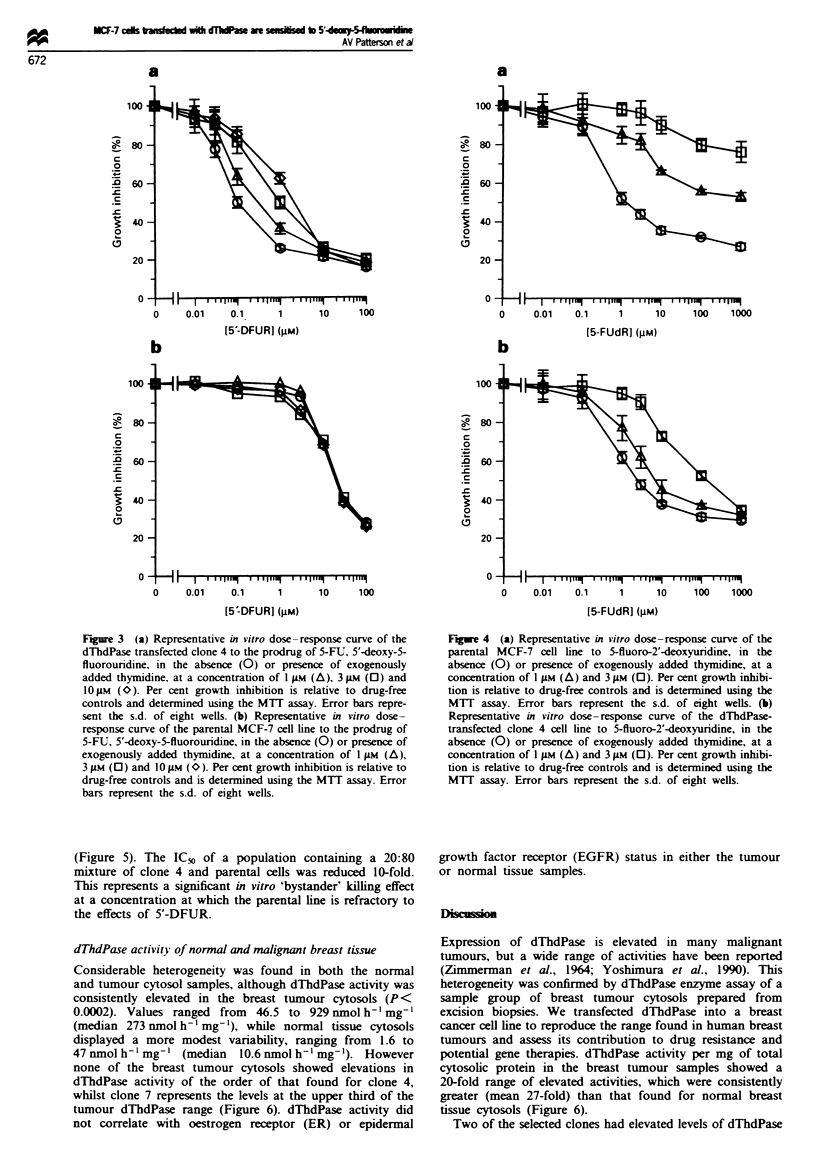
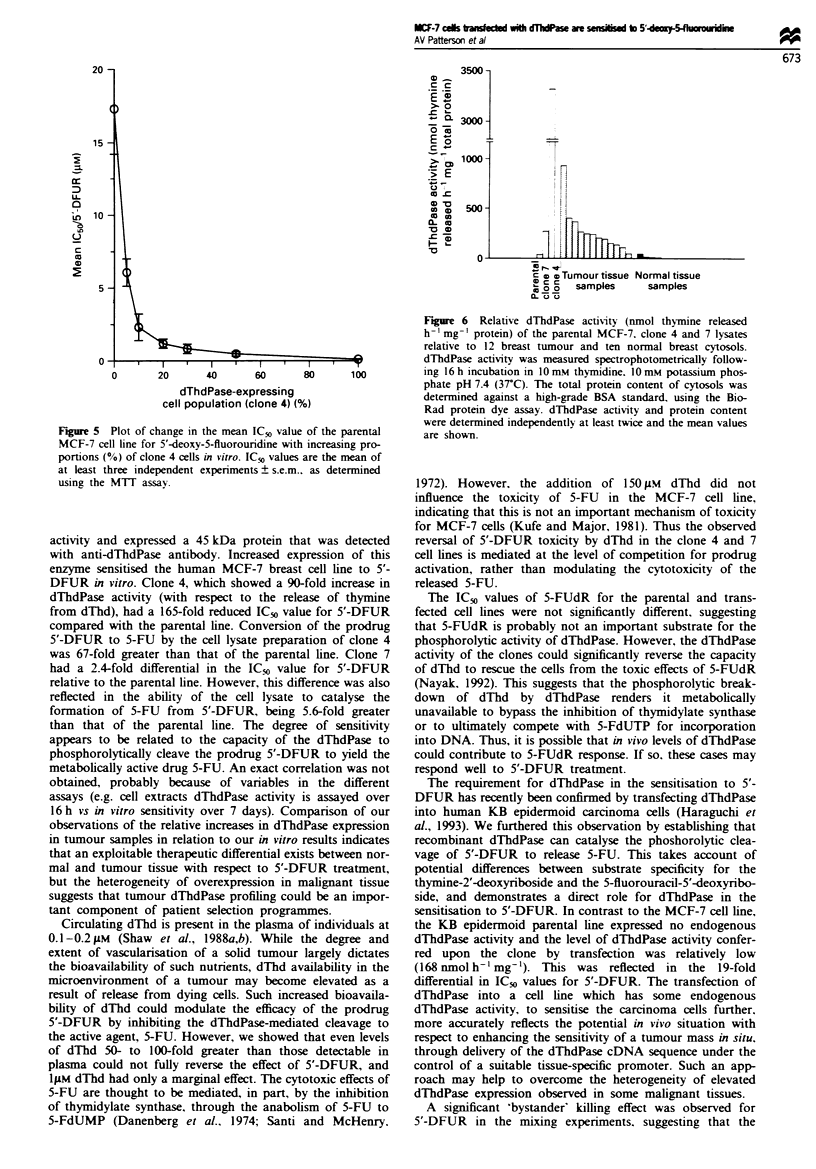
Platelet-derived endothelial cell growth factor (PD-ECGF) is identical to human thymidine phosphorylase (dThdPase). The human MCF-7 breast cancer cell line was transfected with the dThdPase cDNA and expressed a 45 kDa protein that was detected with anti-dThdPase antibody. Cell lysates possessed elevated dThdPase activity and cells had up to 165-fold increased sensitivity to the prodrug 5'-deoxy-5-fluorouridine (5'-DFUR) in vitro. Sensitivity to 5-fluorouracil (5-FU) and 5-fluoro-2'-deoxyuridine (5-FUdR) was unchanged. Recombinant dThdPase was shown to catalyse directly the phosphorolytic cleavage of 5'-DFUR to 5-FU. Exogenous thymidine (dThd) reversed the toxicity of 5-FUdR on the parental line (1 microM dThd increased the IC50 value 1000-fold), but the dThd rescue was substantially modulated in the dThdPase-expressing clone 4 (1 microM dThd raised the IC50 value 3-fold). We observed a substantial 'bystander' killing effect when small proportions of dThdPase-expressing cells were mixed with parental MCF-7 cells. dThdPase activity was on average 27-fold higher in breast tumours than in normal breast. The levels of wild-type MCF-7 are similar to the low end of the tumour expression. Thus, in some tumours resistance to 5'-DFUR therapy could be due to low dThdPase activity, and transfection to raise the dThdPase levels within the broad tumour range or above it should markedly enhance sensitivity to the prodrug. These results confirm that dThdPase is a major pathway in the metabolic activation of 5'-DFUR, and the bystander effect suggests that this may be a suitable enzyme for gene therapy-directed enzyme/prodrug activation therapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram J. S. Fifth Heidelberger Conference on Targets for Cancer Research: prevention, differentiation, and selective therapy. Cancer Res. 1995 Feb 1;55(3):705–709. [PubMed] [Google Scholar]
- Bi W. L., Parysek L. M., Warnick R., Stambrook P. J. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther. 1993 Dec;4(6):725–731. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Choong Y. S., Lee S. P. The degradation of 5'-deoxy-5-fluorouridine by pyrimidine nucleoside phosphorylase in normal and cancer tissues. Clin Chim Acta. 1985 Jul 15;149(2-3):175–183. doi: 10.1016/0009-8981(85)90331-6. [DOI] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano R. J., Smith L. C., Woo S. L. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2122–2126. doi: 10.1073/pnas.90.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg P. V., Langenbach R. J., Heidelberger C. Structures of reversible and irreversible complexes of thymidylate synthetase and fluorinated pyrimidine nucleotides. Biochemistry. 1974 Feb 26;13(5):926–933. doi: 10.1021/bi00702a016. [DOI] [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Fujimoto S., Wang Y., Inoue K., Ogawa M. Antitumor activity of a new fluoropyrimidine derivative, 5'-deoxy-5-fluorouridine, against murine and human experimental tumors. Jpn J Cancer Res. 1985 Jul;76(7):644–650. [PubMed] [Google Scholar]
- Haraguchi M., Furukawa T., Sumizawa T., Akiyama S. Sensitivity of human KB cells expressing platelet-derived endothelial cell growth factor to pyrimidine antimetabolites. Cancer Res. 1993 Dec 1;53(23):5680–5682. [PubMed] [Google Scholar]
- Houghton J. A., Morton C. L., Adkins D. A., Rahman A. Locus of the interaction among 5-fluorouracil, leucovorin, and interferon-alpha 2a in colon carcinoma cells. Cancer Res. 1993 Sep 15;53(18):4243–4250. [PubMed] [Google Scholar]
- Huber B. E., Austin E. A., Good S. S., Knick V. C., Tibbels S., Richards C. A. In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer Res. 1993 Oct 1;53(19):4619–4626. [PubMed] [Google Scholar]
- Iltzsch M. H., el Kouni M. H., Cha S. Kinetic studies of thymidine phosphorylase from mouse liver. Biochemistry. 1985 Nov 19;24(24):6799–6807. doi: 10.1021/bi00345a011. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A. Pentosyl transfer mechanisms of the mammalian nucleoside phosphorylases. J Biol Chem. 1968 Jun 10;243(11):2871–2875. [PubMed] [Google Scholar]
- Kufe D. W., Major P. P. 5-Fluorouracil incorporation into human breast carcinoma RNA correlates with cytotoxicity. J Biol Chem. 1981 Oct 10;256(19):9802–9805. [PubMed] [Google Scholar]
- Major P. P., Egan E., Herrick D., Kufe D. W. 5-Fluorouracil incorporation in DNA of human breast carcinoma cells. Cancer Res. 1982 Aug;42(8):3005–3009. [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. High-yield purification of platelet-derived endothelial cell growth factor: structural characterization and establishment of a specific antiserum. Biochemistry. 1989 Feb 21;28(4):1704–1710. doi: 10.1021/bi00430a042. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Okabe T., Urabe A., Takaku F., Heldin C. H. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. 1987 Mar 25;262(9):4098–4103. [PubMed] [Google Scholar]
- Moghaddam A., Bicknell R. Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry. 1992 Dec 8;31(48):12141–12146. doi: 10.1021/bi00163a024. [DOI] [PubMed] [Google Scholar]
- Nayak R. Thymidine inhibits the incorporation of 5-fluoro-2'-deoxyuridine to DNA of mouse mammary tumour. Biochem Biophys Res Commun. 1992 Apr 15;184(1):467–470. doi: 10.1016/0006-291x(92)91217-e. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Paolini N. S., Ebarb R. L., Germain M. J. Elevated thymidine phosphorylase activity in the plasma and ascitis fluids of tumor-bearing animals. Proc Soc Exp Biol Med. 1978 Feb;157(2):262–267. doi: 10.3181/00379727-157-40034. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Schuller M. G., Zelcer A. A., Kirss T. A., Gore S. S., Germain M. J. Identification and comparative analysis of thymidine phosphorylase in the plasma of healthy subjects and cancer patients. J Natl Cancer Inst. 1977 Jun;58(6):1587–1590. doi: 10.1093/jnci/58.6.1587. [DOI] [PubMed] [Google Scholar]
- Santelli G., Valeriote F. In vivo potentiation of 5-fluorouracil cytotoxicity against AKR leukemia by purines, pyrimidines, and their nucleosides and deoxynucleosides. J Natl Cancer Inst. 1980 Jan;64(1):69–72. [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S. 5-Fluoro-2'-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1855–1857. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. L., Baptiste N., O'Connor C. J., Wadler S., Otter B. A. Potentiation of the antitumor activity of 5-fluorouracil in colon carcinoma cells by the combination of interferon and deoxyribonucleosides results from complementary effects on thymidine phosphorylase. Cancer Res. 1994 Mar 15;54(6):1472–1478. [PubMed] [Google Scholar]
- Schwartz E. L., Hoffman M., O'Connor C. J., Wadler S. Stimulation of 5-fluorouracil metabolic activation by interferon-alpha in human colon carcinoma cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1232–1239. doi: 10.1016/0006-291x(92)91863-l. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Thymidine phosphorylase from Escherichia coli. Methods Enzymol. 1978;51:442–445. doi: 10.1016/s0076-6879(78)51061-6. [DOI] [PubMed] [Google Scholar]
- Shaw T., Smillie R. H., Miller A. E., MacPhee D. G. The role of blood platelets in nucleoside metabolism: regulation of platelet thymidine phosphorylase. Mutat Res. 1988 Jul-Aug;200(1-2):117–131. doi: 10.1016/0027-5107(88)90075-9. [DOI] [PubMed] [Google Scholar]
- Sobrero A. F., Aschele C., Guglielmi A. P., Mori A. M., Melioli G. G., Rosso R., Bertino J. R. Synergism and lack of cross-resistance between short-term and continuous exposure to fluorouracil in human colon adenocarcinoma cells. J Natl Cancer Inst. 1993 Dec 1;85(23):1937–1944. doi: 10.1093/jnci/85.23.1937. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Eda H., Fujimoto K., Tanaka T., Ishikawa T., Ishitsuka H. Anticachectic activity of 5'-deoxy-5-fluorouridine in a murine tumor cachexia model, colon 26 adenocarcinoma. Cancer Res. 1990 Aug 1;50(15):4528–4532. [PubMed] [Google Scholar]
- Vile R. G., Hart I. R. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 1993 Mar 1;53(5):962–967. [PubMed] [Google Scholar]
- Vile R. G., Hart I. R. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 1993 Sep 1;53(17):3860–3864. [PubMed] [Google Scholar]
- Yoshimura A., Kuwazuru Y., Furukawa T., Yoshida H., Yamada K., Akiyama S. Purification and tissue distribution of human thymidine phosphorylase; high expression in lymphocytes, reticulocytes and tumors. Biochim Biophys Acta. 1990 Apr 23;1034(1):107–113. doi: 10.1016/0304-4165(90)90160-x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN M., SEIDENBERG J. DEOXYRIBOSYL TRANSFER. I. THYMIDINE PHOSPHORYLASE AND NUCLEOSIDE DEOXYRIBOSYLTRANSFERASE IN NORMAL AND MALIGNANT TISSUES. J Biol Chem. 1964 Aug;239:2618–2621. [PubMed] [Google Scholar]
- el Kouni M. H., el Kouni M. M., Naguib F. N. Differences in activities and substrate specificity of human and murine pyrimidine nucleoside phosphorylases: implications for chemotherapy with 5-fluoropyrimidines. Cancer Res. 1993 Aug 15;53(16):3687–3693. [PubMed] [Google Scholar]



