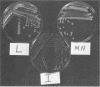
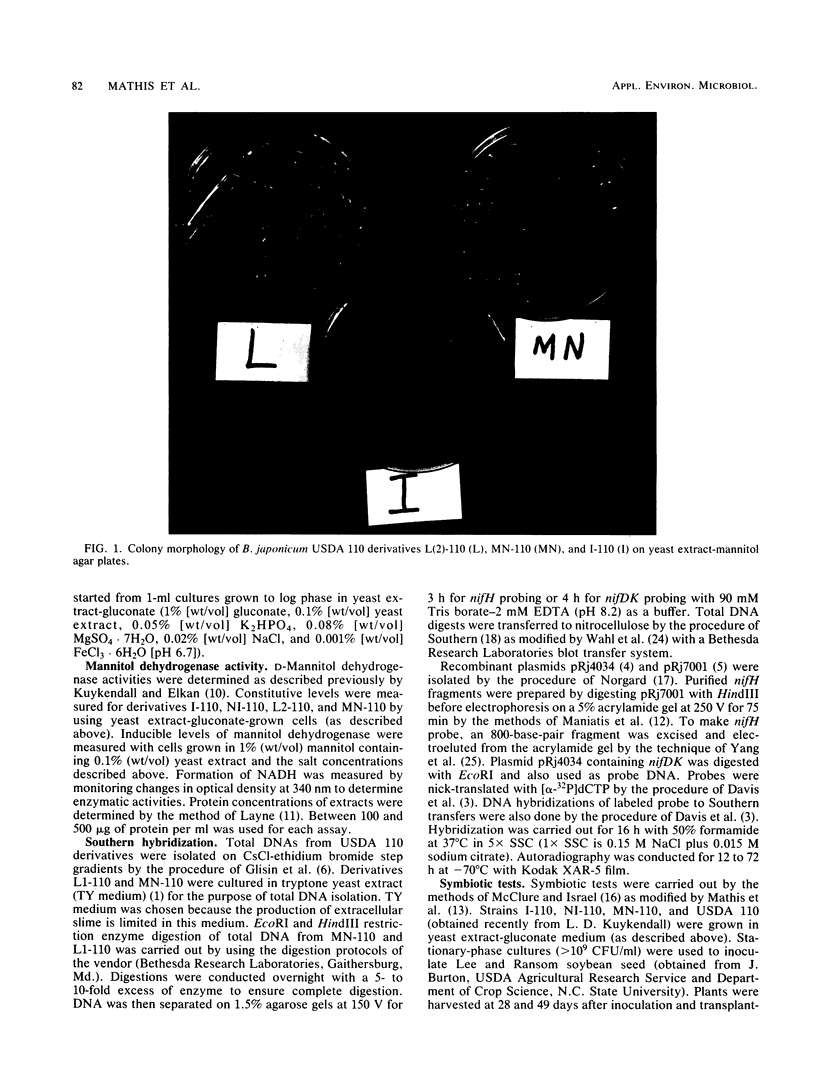
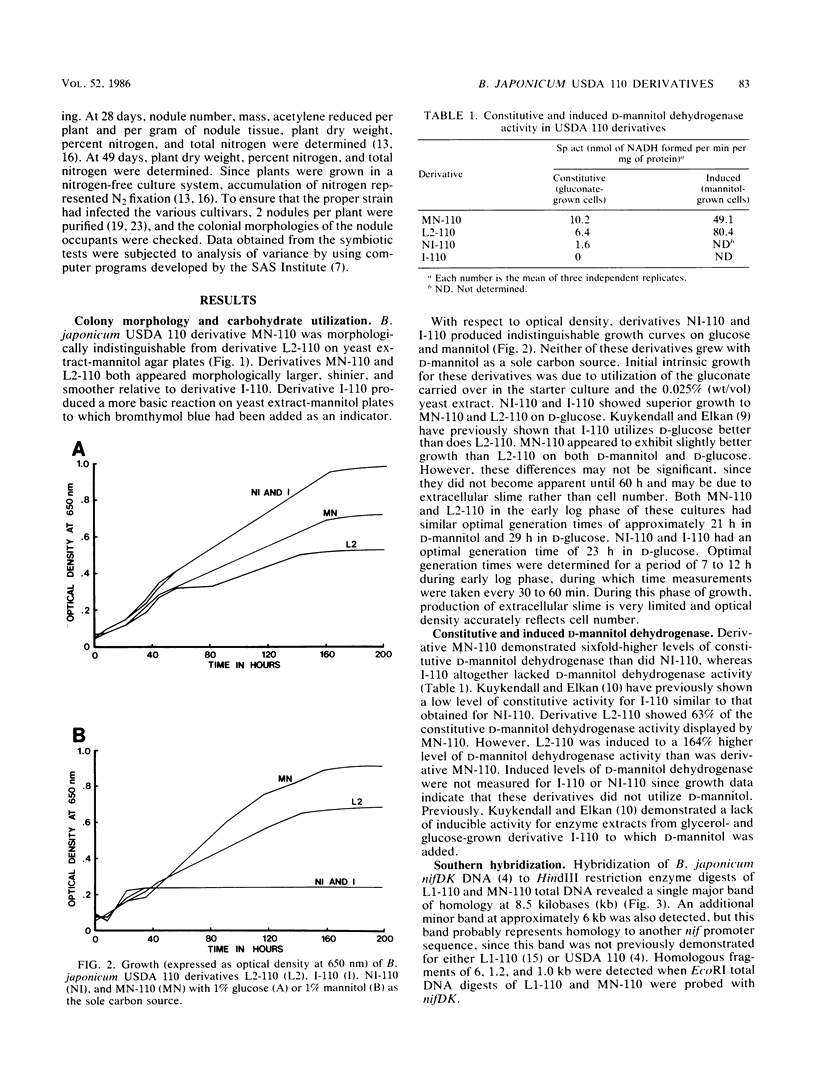
Abstract
We have isolated a colonial derivative of Bradyrhizobium japonicum USDA 110 (designated MN-110) that is both mannitol utilizing and N2 fixing. Derivative MN-110 showed growth on mannitol and glucose similar to that of non-N2-fixing, mannitol-utilizing L2-110. Derivative MN-110 showed high constitutive and induced d-mannitol dehydrogenase activity (similar to L2-110) relative to N2-fixing, non-mannitol-utilizing I-110. Hybridization to EcoRI and HindIII total DNA digests with cloned USDA 110 nif DK and nif H genes revealed similar patterns for non-N2-fixing mannitol-utilizing derivative L1-110 and derivative MN-110. Symbiotic tests with soybean cultivars Ransom and Lee indicate MN-110 to be a superior N2-fixing derivative compared with derivative I-110 and the parent strain USDA 110. However, these differences were not revealed when comparing 28-day-old soybean-B. japonicum associations but were apparent in 49-day-old associations. It was apparent from this work that mannitol utilization was not necessarily correlated to symbiotic effectiveness in B. japonicum and that gene rearrangements were not responsible for differences in N2 fixation between L1-110 or L2-110 and MN-110.
Full text
PDF
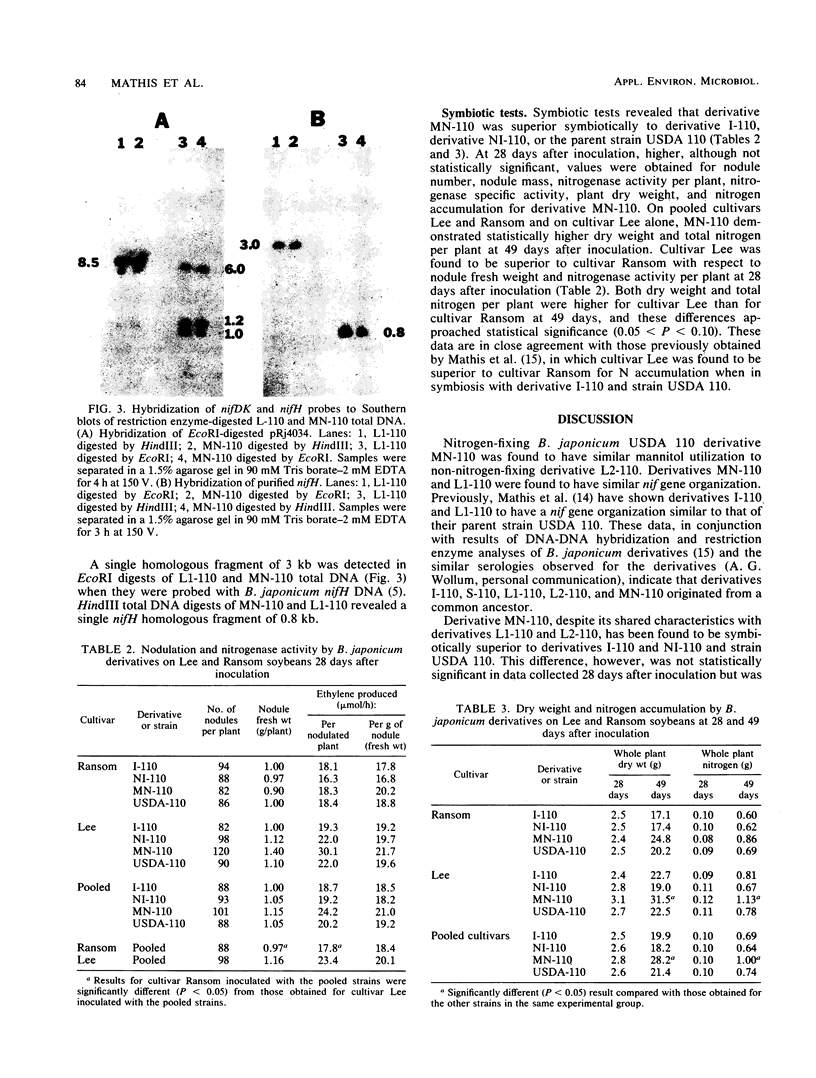

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M., Hennecke H. Rhizobium japonicum nitrogenase Fe protein gene (nifH). J Bacteriol. 1984 Jun;158(3):1005–1011. doi: 10.1128/jb.158.3.1005-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Herridge D. F., Roughley R. J. Variation in colony characteristics and symbiotic effectiveness of Rhizobium. J Appl Bacteriol. 1975 Feb;38(1):19–27. doi: 10.1111/j.1365-2672.1975.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Some features of mannitol metabolism in Rhizobium japonicum. J Gen Microbiol. 1977 Jan;98(1):291–295. doi: 10.1099/00221287-98-1-291. [DOI] [PubMed] [Google Scholar]
- Mathis J. N., Barbour W. M., Elkan G. H. Effect of Sym Plasmid Curing on Symbiotic Effectiveness in Rhizobium fredii. Appl Environ Microbiol. 1985 Jun;49(6):1385–1388. doi: 10.1128/aem.49.6.1385-1388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. N., Israel D. W., Barbour W. M., Jarvis B. D., Elkan G. H. Analysis of the Symbiotic Performance of Bradyrhizobium japonicum USDA 110 and Its Derivative I-110 and Discovery of a New Mannitol-Utilizing, Nitrogen-Fixing USDA 110 Derivative. Appl Environ Microbiol. 1986 Jul;52(1):75–80. doi: 10.1128/aem.52.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. N., Kuykendall L. D., Elkan G. H. Restriction Endonuclease and nif Homology Patterns of Bradyrhizobium japonicum USDA 110 Derivatives With and Without Nitrogen Fixation Competence. Appl Environ Microbiol. 1986 Mar;51(3):477–480. doi: 10.1128/aem.51.3.477-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V. Rapid and simple removal of contaminating RNA from plasmid DNA without the use of RNase. Anal Biochem. 1981 May 1;113(1):34–42. doi: 10.1016/0003-2697(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Upchurch R. G., Elkan G. H. Comparison of colony morphology, salt tolerance, and effectiveness in Rhizobium japonicum. Can J Microbiol. 1977 Sep;23(9):1118–1122. doi: 10.1139/m77-167. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]