Abstract
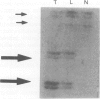
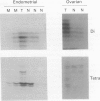
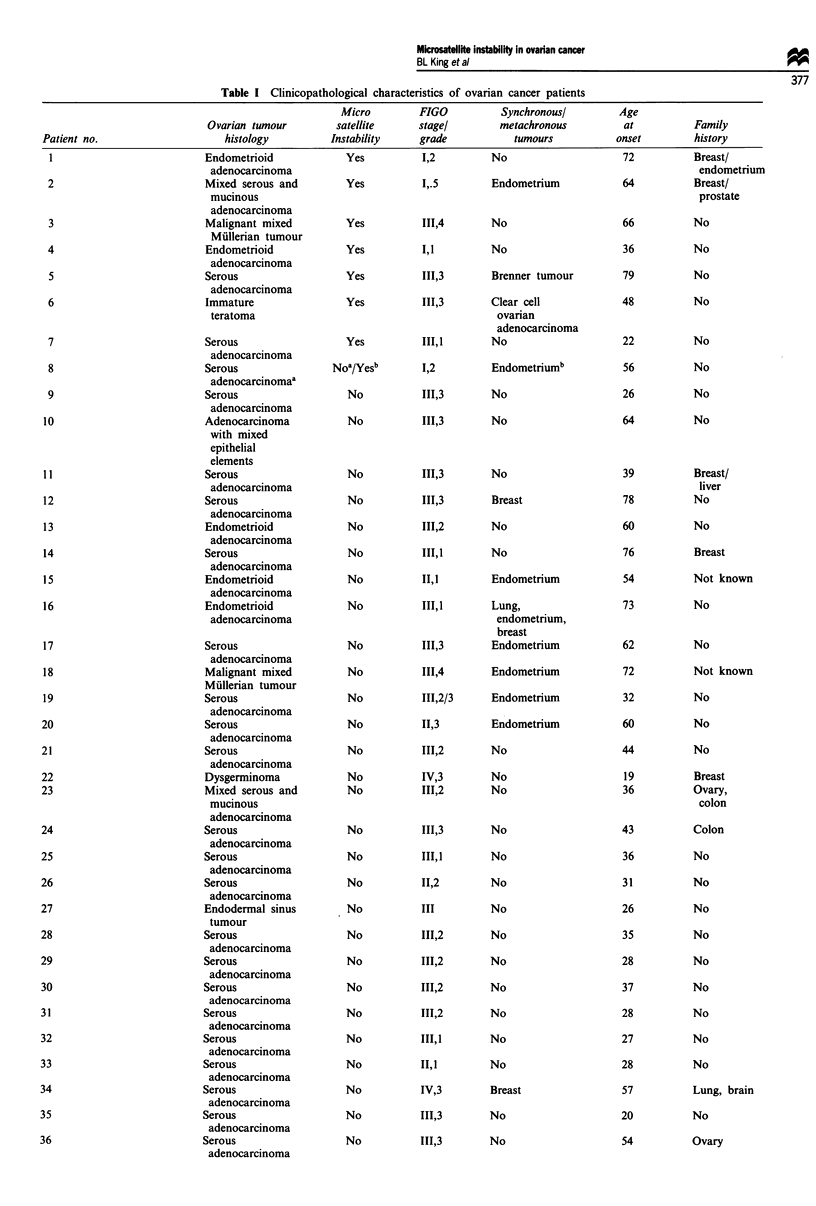
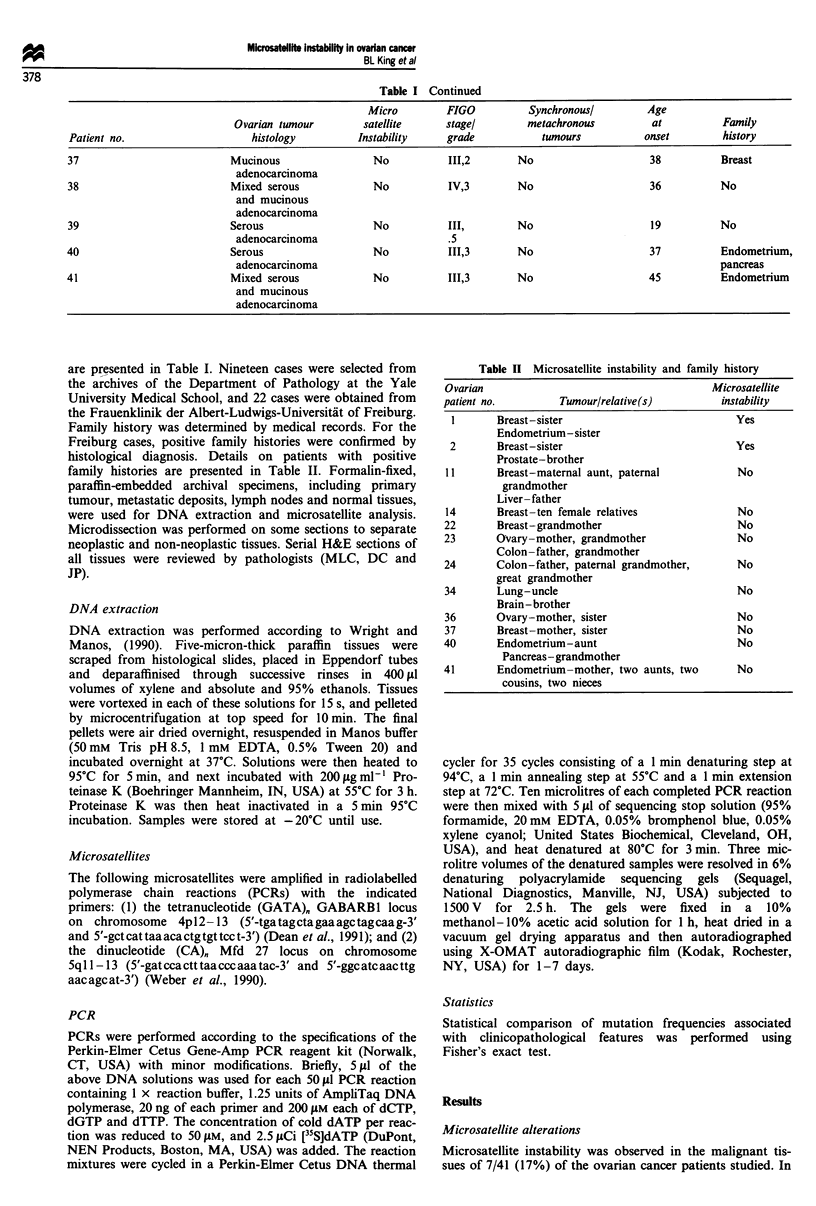
Microsatellite instability has been observed in a variety of sporadic malignancies, but its existence in sporadic ovarian cancer has been the subject of conflicting reports. We have performed a polymerase chain reaction-based microsatellite analysis of DNAs extracted from the neoplastic and non-neoplastic tissues of 41 ovarian cancer patients. Tumour-associated alterations were observed in seven (17%) of these cases. Clinicopathological correlations revealed that: (1) alterations among tumours classified as serous adenocarcinomas occurred with relatively low frequency (2/24 or 8%); (2) most of the tumours with microsatellite alterations (5/7 or 71%) were of less common histopathological types (epithelial subtypes such as endometrioid and mixed serous and mucinous, or non-epithelial types such as malignant mixed Müllerian or germ cell tumours); (3) tumour-associated alterations were observed in 3/4 (75%) of the patients with stage I tumours vs 4/37 (11%) of the patients with stage II, III and IV tumours (P = 0.01); (4) tumour-associated microsatellite instability was found to occur with similar frequencies among patients with and without clinical features suggestive of familial disease, including positive family history, early onset, or multiple primary tumours. In summary, we have observed microsatellite alterations in the neoplastic tissues of ovarian cancer patients with diverse genetic backgrounds and clinicopathological features. The pattern of alterations is consistent with the possibility that multiple mechanisms may be responsible for microsatellite instability in ovarian neoplasms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Dean M., Lucas-Derse S., Bolos A., O'Brien S. J., Kirkness E. F., Fraser C. M., Goldman D. Genetic mapping of the beta 1 GABA receptor gene to human chromosome 4, using a tetranucleotide repeat polymorphism. Am J Hum Genet. 1991 Sep;49(3):621–626. [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Piver M. S. The genetics of ovarian cancer. Cancer Invest. 1992;10(2):135–141. doi: 10.3109/07357909209032774. [DOI] [PubMed] [Google Scholar]
- Duggan B. D., Felix J. C., Muderspach L. I., Tourgeman D., Zheng J., Shibata D. Microsatellite instability in sporadic endometrial carcinoma. J Natl Cancer Inst. 1994 Aug 17;86(16):1216–1221. doi: 10.1093/jnci/86.16.1216. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Ruppert J. M., Tokino K., Tsai Y. C., Spruck C. H., 3rd, Miyao N., Nichols P. W., Hermann G. G., Horn T., Steven K. Microsatellite instability in bladder cancer. Cancer Res. 1993 Dec 1;53(23):5620–5623. [PubMed] [Google Scholar]
- Han H. J., Maruyama M., Baba S., Park J. G., Nakamura Y. Genomic structure of human mismatch repair gene, hMLH1, and its mutation analysis in patients with hereditary non-polyposis colorectal cancer (HNPCC) Hum Mol Genet. 1995 Feb;4(2):237–242. doi: 10.1093/hmg/4.2.237. [DOI] [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- King B. L., Lichtenstein A., Berenson J., Kacinski B. M. A polymerase chain reaction-based microsatellite typing assay used for tumor cell line identification. Am J Pathol. 1994 Mar;144(3):486–491. [PMC free article] [PubMed] [Google Scholar]
- Kolodner R. D., Hall N. R., Lipford J., Kane M. F., Morrison P. T., Finan P. J., Burn J., Chapman P., Earabino C., Merchant E. Structure of the human MLH1 locus and analysis of a large hereditary nonpolyposis colorectal carcinoma kindred for mlh1 mutations. Cancer Res. 1995 Jan 15;55(2):242–248. [PubMed] [Google Scholar]
- Kolodner R. D., Hall N. R., Lipford J., Kane M. F., Rao M. R., Morrison P., Wirth L., Finan P. J., Burn J., Chapman P. Structure of the human MSH2 locus and analysis of two Muir-Torre kindreds for msh2 mutations. Genomics. 1994 Dec;24(3):516–526. doi: 10.1006/geno.1994.1661. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R. E., Hamilton S. R., Petersen G. M., Lynch H. T., Watson P., Markowitz S., Willson J. K., Green J., de la Chapelle A. hMSH2 mutations in hereditary nonpolyposis colorectal cancer kindreds. Cancer Res. 1994 Sep 1;54(17):4590–4594. [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lynch H. T., Bewtra C., Lynch J. F. Familial ovarian carcinoma. Clinical nuances. Am J Med. 1986 Dec;81(6):1073–1076. doi: 10.1016/0002-9343(86)90411-0. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Drouhard T., Lanspa S., Smyrk T., Lynch P., Lynch J., Vogelstein B., Nyström-Lahti M., Sistonen P., Peltomäki P. Mutation of an mutL homologue in a Navajo family with hereditary nonpolyposis colorectal cancer. J Natl Cancer Inst. 1994 Sep 21;86(18):1417–1419. doi: 10.1093/jnci/86.18.1417. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. A polymorphic X-linked tetranucleotide repeat locus displaying a high rate of new mutation: implications for mechanisms of mutation at short tandem repeat loci. Hum Mol Genet. 1993 Apr;2(4):431–437. doi: 10.1093/hmg/2.4.431. [DOI] [PubMed] [Google Scholar]
- Mao L., Lee D. J., Tockman M. S., Erozan Y. S., Askin F., Sidransky D. Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9871–9875. doi: 10.1073/pnas.91.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary J. L., Bishop T., Kolodner R., Lipford J. R., Kane M., Weber W., Torhorst J., Müller H., Spycher M., Scott R. J. Mutational analysis of the hMSH2 gene reveals a three base pair deletion in a family predisposed to colorectal cancer development. Hum Mol Genet. 1994 Nov;3(11):2067–2069. [PubMed] [Google Scholar]
- Meltzer S. J., Yin J., Manin B., Rhyu M. G., Cottrell J., Hudson E., Redd J. L., Krasna M. J., Abraham J. M., Reid B. J. Microsatellite instability occurs frequently and in both diploid and aneuploid cell populations of Barrett's-associated esophageal adenocarcinomas. Cancer Res. 1994 Jul 1;54(13):3379–3382. [PubMed] [Google Scholar]
- Merlo A., Mabry M., Gabrielson E., Vollmer R., Baylin S. B., Sidransky D. Frequent microsatellite instability in primary small cell lung cancer. Cancer Res. 1994 Apr 15;54(8):2098–2101. [PubMed] [Google Scholar]
- Mironov N. M., Aguelon M. A., Potapova G. I., Omori Y., Gorbunov O. V., Klimenkov A. A., Yamasaki H. Alterations of (CA)n DNA repeats and tumor suppressor genes in human gastric cancer. Cancer Res. 1994 Jan 1;54(1):41–44. [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Orth K., Hung J., Gazdar A., Bowcock A., Mathis J. M., Sambrook J. Genetic instability in human ovarian cancer cell lines. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9495–9499. doi: 10.1073/pnas.91.20.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R. J., Leech V. Polymerase chain reaction allelotyping of human ovarian cancer. Br J Cancer. 1994 Mar;69(3):429–438. doi: 10.1038/bjc.1994.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M. J., Fang W. H., Papadopoulos N., Jen J., de la Chapelle A., Kinzler K. W., Vogelstein B., Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993 Dec 17;75(6):1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Lothe R. A., Aaltonen L. A., Pylkkänen L., Nyström-Lahti M., Seruca R., David L., Holm R., Ryberg D., Haugen A. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993 Dec 15;53(24):5853–5855. [PubMed] [Google Scholar]
- Radman M., Wagner R. Carcinogenesis. Missing mismatch repair. Nature. 1993 Dec 23;366(6457):722–722. doi: 10.1038/366722a0. [DOI] [PubMed] [Google Scholar]
- Rhyu M. G., Park W. S., Meltzer S. J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994 Jan;9(1):29–32. [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Shridhar V., Siegfried J., Hunt J., del Mar Alonso M., Smith D. I. Genetic instability of microsatellite sequences in many non-small cell lung carcinomas. Cancer Res. 1994 Apr 15;54(8):2084–2087. [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Uchida T., Wada C., Wang C., Egawa S., Ohtani H., Koshiba K. Genomic instability of microsatellite repeats and mutations of H-, K-, and N-ras, and p53 genes in renal cell carcinoma. Cancer Res. 1994 Jul 15;54(14):3682–3685. [PubMed] [Google Scholar]
- Umar A., Boyer J. C., Kunkel T. A. DNA loop repair by human cell extracts. Science. 1994 Nov 4;266(5186):814–816. doi: 10.1126/science.7973637. [DOI] [PubMed] [Google Scholar]
- Umar A., Boyer J. C., Thomas D. C., Nguyen D. C., Risinger J. I., Boyd J., Ionov Y., Perucho M., Kunkel T. A. Defective mismatch repair in extracts of colorectal and endometrial cancer cell lines exhibiting microsatellite instability. J Biol Chem. 1994 May 20;269(20):14367–14370. [PubMed] [Google Scholar]
- Wada C., Shionoya S., Fujino Y., Tokuhiro H., Akahoshi T., Uchida T., Ohtani H. Genomic instability of microsatellite repeats and its association with the evolution of chronic myelogenous leukemia. Blood. 1994 Jun 15;83(12):3449–3456. [PubMed] [Google Scholar]
- Watson P., Lynch H. T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993 Feb 1;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E. Dinucleotide repeat polymorphisms at the D5S107, D5S108, D5S111, D5S117 and D5S118 loci. Nucleic Acids Res. 1990 Jul 11;18(13):4035–4035. doi: 10.1093/nar/18.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., Wong C. Mutation of human short tandem repeats. Hum Mol Genet. 1993 Aug;2(8):1123–1128. doi: 10.1093/hmg/2.8.1123. [DOI] [PubMed] [Google Scholar]
- Wooster R., Cleton-Jansen A. M., Collins N., Mangion J., Cornelis R. S., Cooper C. S., Gusterson B. A., Ponder B. A., von Deimling A., Wiestler O. D. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet. 1994 Feb;6(2):152–156. doi: 10.1038/ng0294-152. [DOI] [PubMed] [Google Scholar]