Abstract
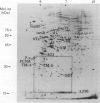
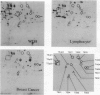
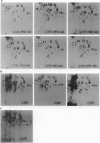
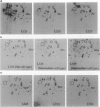
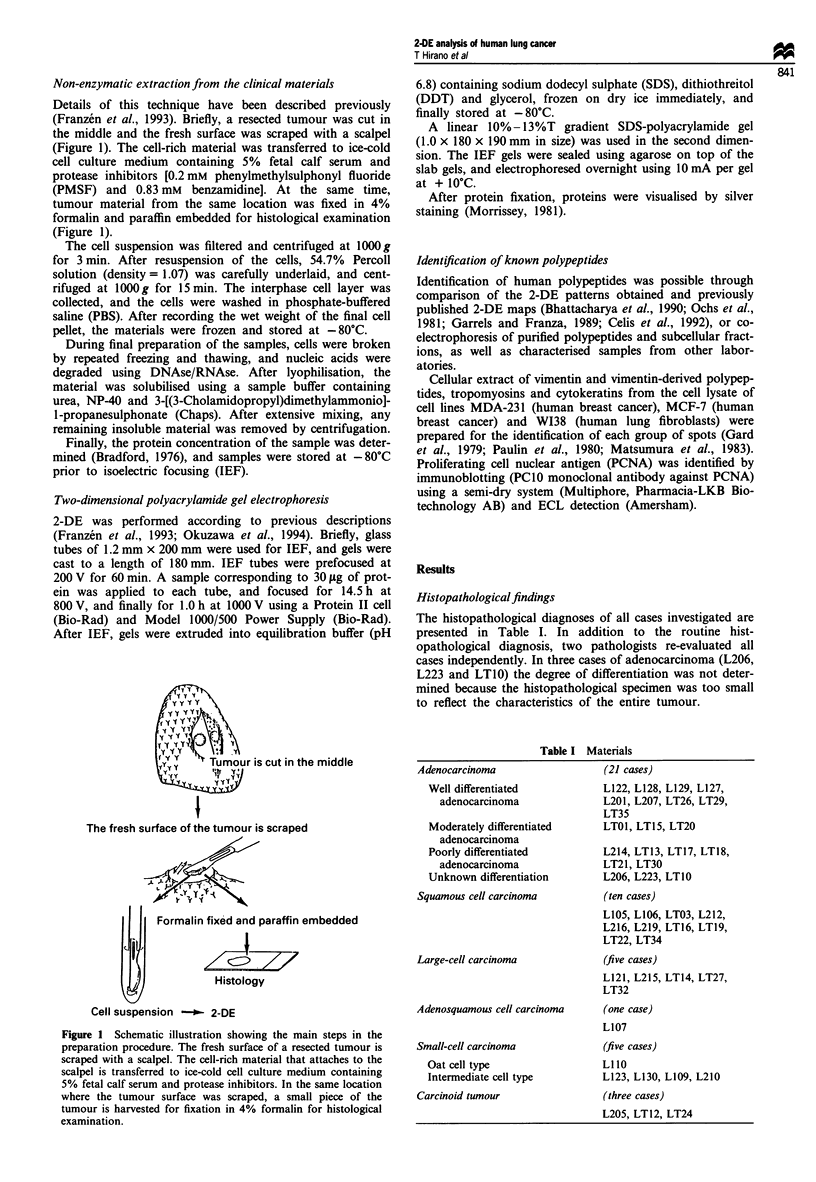
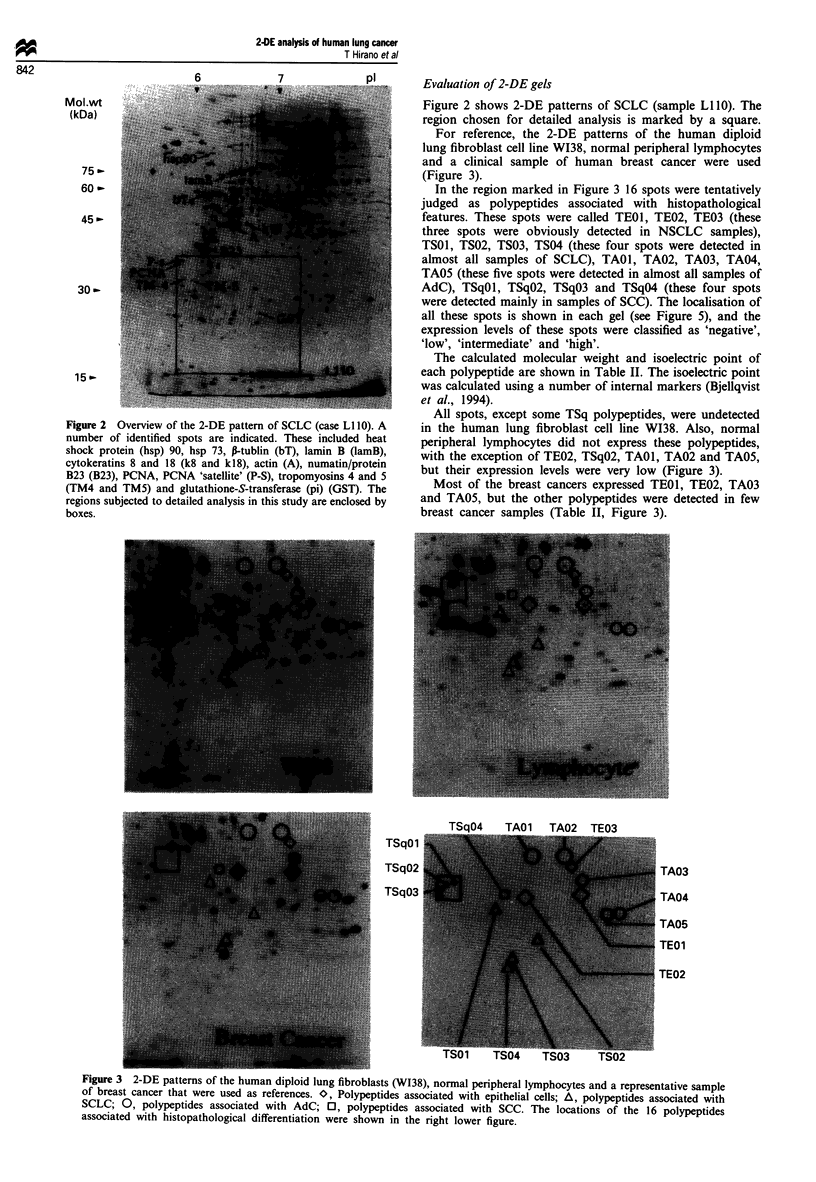
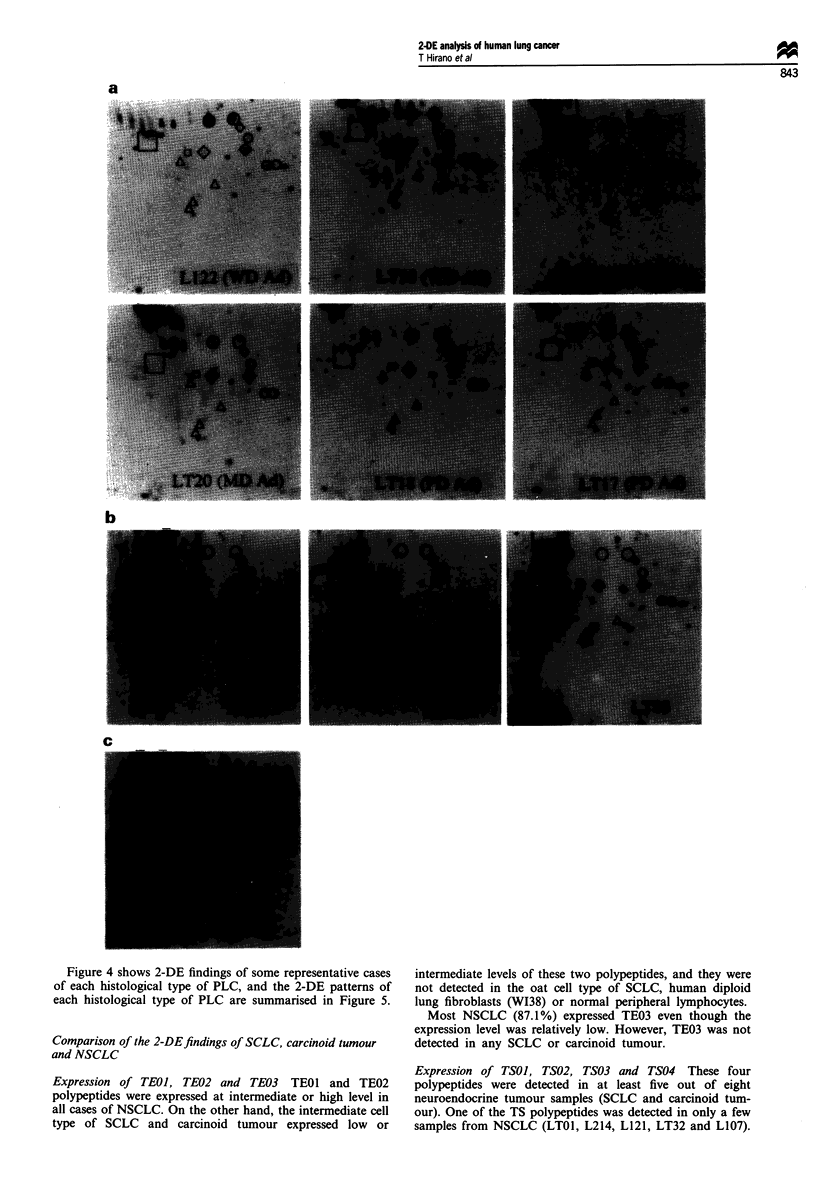
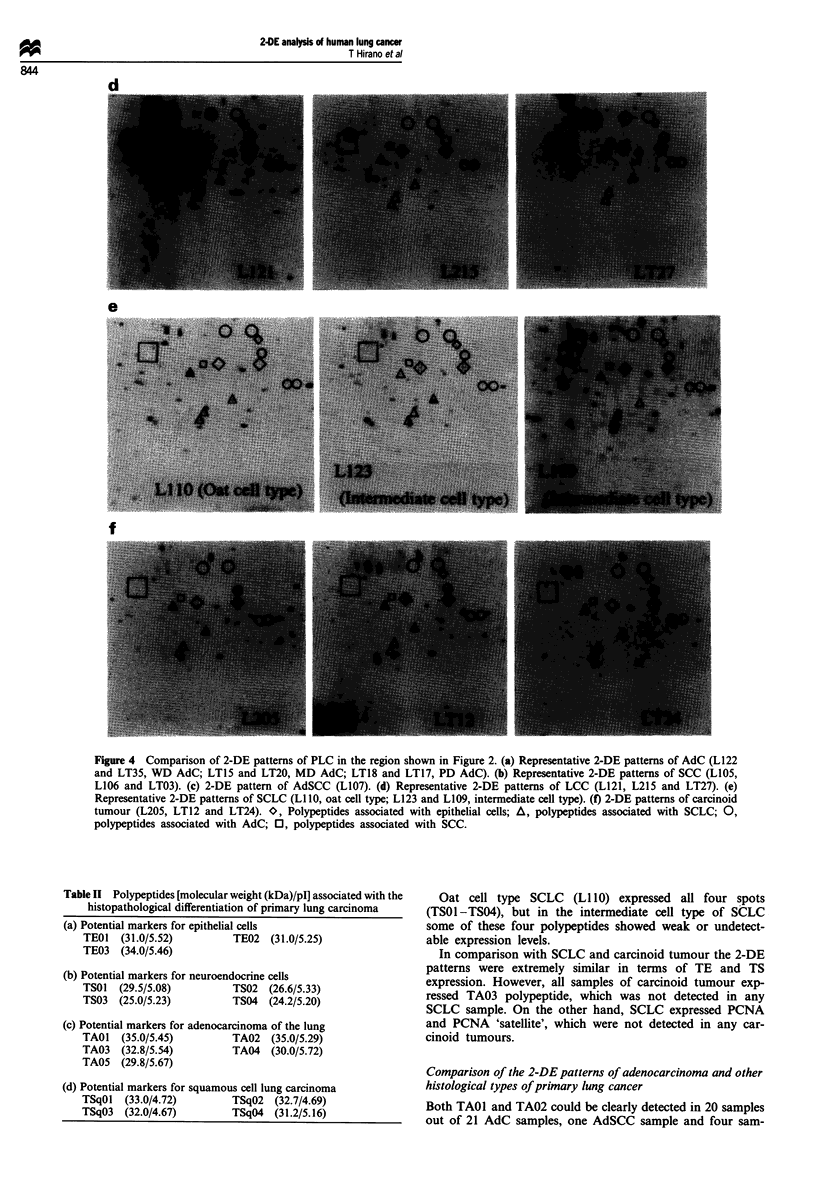
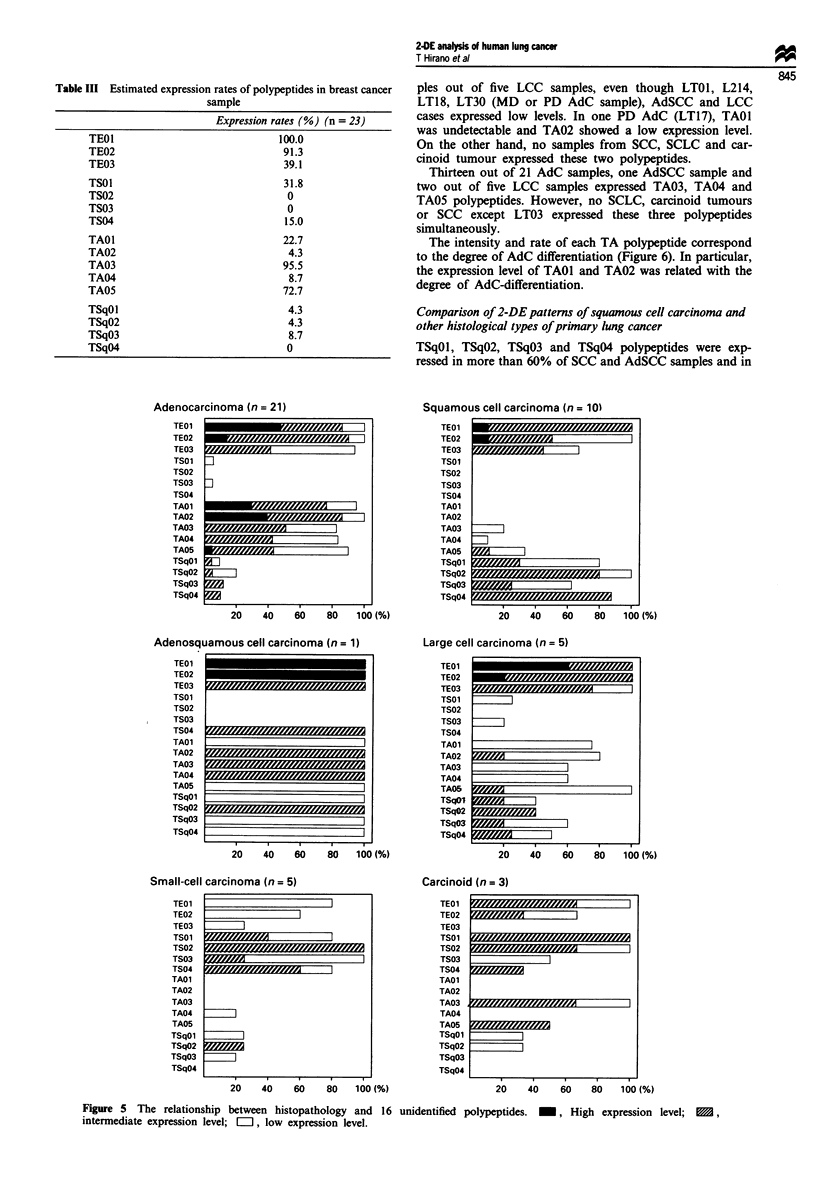
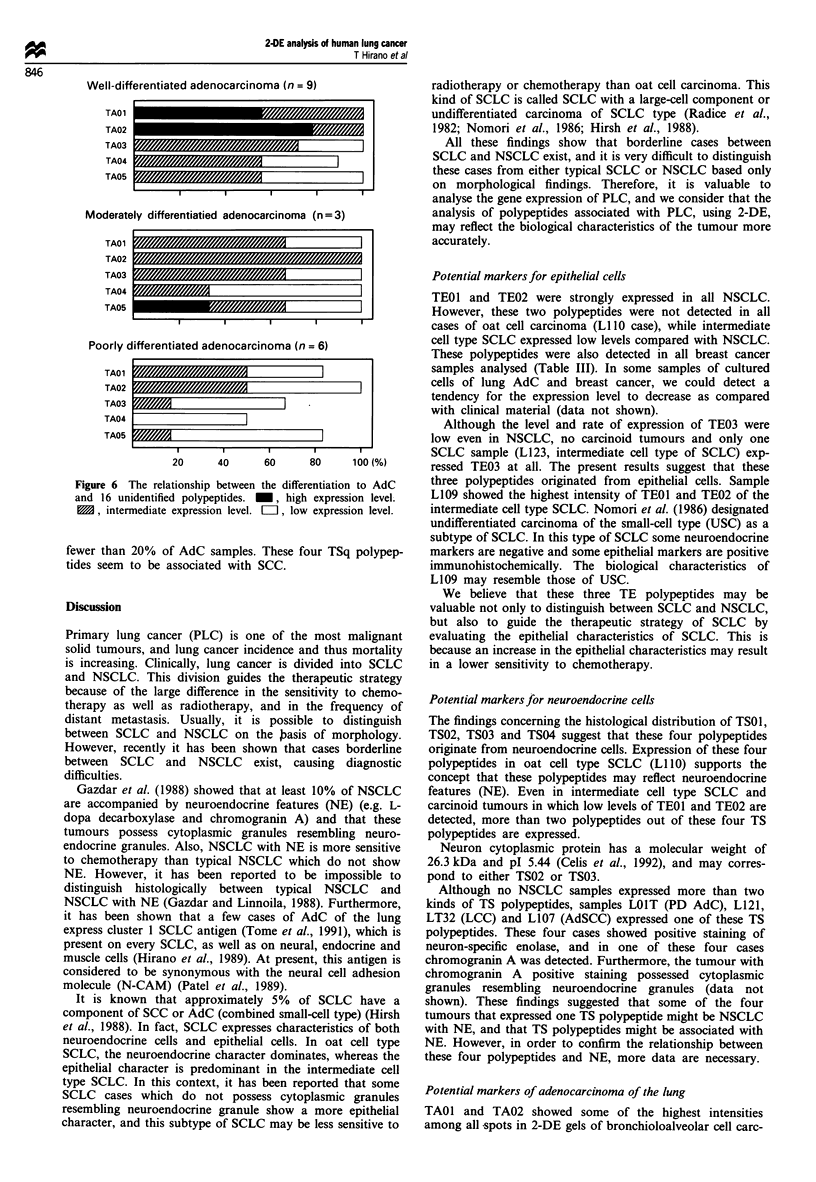
Two-dimensional polyacrylamide gel electrophoresis combined with a non-enzymatic sample preparation technique is useful for analysing clinical tumour material. Using these techniques, we analysed the relationship between the histopathological findings in primary lung malignancies and the expression of a number of unidentified polypeptides that were detected in the molecular weight region 20-35 kDa. In this study 45 cases of primary lung cancer (PLC) (21 cases of adenocarcinoma, ten cases of squamous cell carcinoma, five cases of large-cell carcinoma, one case of adenosquamous cell carcinoma, five cases of small-cell carcinoma and three cases of carcinoid tumour) were examined. For reference, a human diploid fibroblast cell line (W138) and normal peripheral lymphocytes were used. Sixteen polypeptides were judged to be associated with histopathological features. These polypeptides seem to be valuable as differentiation markers. The simultaneous evaluation of these polypeptides and some other proliferation markers (e.g. PCNA, PCNA 'satellite', Numatin/protein B23 and lamin B) seems to clarify the characteristics of each case of PLC. Furthermore, it is possible to classify PLC based on the two-dimensional electrophoresis findings, and this classification of PLC is suggested to reflect the biological features of the tumour more precisely than that based only on morphology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer G., Ono J., Nasiell M., Caspersson T., Kato H., Konaka C., Hayata Y. Reversibility of bronchial cell atypia. Cancer Res. 1982 Oct;42(10):4241–4247. [PubMed] [Google Scholar]
- Bhattacharya B., Prasad G. L., Valverius E. M., Salomon D. S., Cooper H. L. Tropomyosins of human mammary epithelial cells: consistent defects of expression in mammary carcinoma cell lines. Cancer Res. 1990 Apr 1;50(7):2105–2112. [PubMed] [Google Scholar]
- Bjellqvist B., Basse B., Olsen E., Celis J. E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis. 1994 Mar-Apr;15(3-4):529–539. doi: 10.1002/elps.1150150171. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Franzén B., Linder S., Okuzawa K., Kato H., Auer G. Nonenzymatic extraction of cells from clinical tumor material for analysis of gene expression by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1993 Oct;14(10):1045–1053. doi: 10.1002/elps.11501401167. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr Transformation-sensitive and growth-related changes of protein synthesis in REF52 cells. A two-dimensional gel analysis of SV40-, adenovirus-, and Kirsten murine sarcoma virus-transformed rat cells using the REF52 protein database. J Biol Chem. 1989 Mar 25;264(9):5299–5312. [PubMed] [Google Scholar]
- Gazdar A. F., Helman L. J., Israel M. A., Russell E. K., Linnoila R. I., Mulshine J. L., Schuller H. M., Park J. G. Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res. 1988 Jul 15;48(14):4078–4082. [PubMed] [Google Scholar]
- Gazdar A. F., Linnoila R. I. The pathology of lung cancer--changing concepts and newer diagnostic techniques. Semin Oncol. 1988 Jun;15(3):215–225. [PubMed] [Google Scholar]
- Hirano T., Franzén B., Kato H., Ebihara Y., Auer G. Genesis of squamous cell lung carcinoma. Sequential changes of proliferation, DNA ploidy, and p53 expression. Am J Pathol. 1994 Feb;144(2):296–302. [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Hirohashi S., Kunii T., Noguchi M., Shimosato Y., Hayata Y. Quantitative distribution of cluster 1 small cell lung cancer antigen in cancerous and non-cancerous tissues, cultured cells and sera. Jpn J Cancer Res. 1989 Apr;80(4):348–355. doi: 10.1111/j.1349-7006.1989.tb02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch F. R., Matthews M. J., Aisner S., Campobasso O., Elema J. D., Gazdar A. F., Mackay B., Nasiell M., Shimosato Y., Steele R. H. Histopathologic classification of small cell lung cancer. Changing concepts and terminology. Cancer. 1988 Sep 1;62(5):973–977. doi: 10.1002/1097-0142(19880901)62:5<973::aid-cncr2820620521>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nasiell M., Carlens E., Auer G., Hayata Y., Kato H., Konaka C., Roger V., Nasiell K., Enstad I. Pathogenesis of bronchial carcinoma, with special reference to morphogenesis and the influence on the bronchial mucosa of 20-methylcholanthrene and cigarette smoking. Recent Results Cancer Res. 1982;82:53–68. doi: 10.1007/978-3-642-81768-7_6. [DOI] [PubMed] [Google Scholar]
- Nomori H., Shimosato Y., Kodama T., Morinaga S., Nakajima T., Watanabe S. Subtypes of small cell carcinoma of the lung: morphometric, ultrastructural, and immunohistochemical analyses. Hum Pathol. 1986 Jun;17(6):604–613. doi: 10.1016/s0046-8177(86)80133-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochs D. C., McConkey E. H., Guard N. L. Vimentin-derived proteins: differences between normal human fibroblasts and transformed human cells. Exp Cell Res. 1981 Oct;135(2):355–362. doi: 10.1016/0014-4827(81)90171-3. [DOI] [PubMed] [Google Scholar]
- Okuzawa K., Franzén B., Lindholm J., Linder S., Hirano T., Bergman T., Ebihara Y., Kato H., Auer G. Characterization of gene expression in clinical lung cancer materials by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1994 Mar-Apr;15(3-4):382–390. doi: 10.1002/elps.1150150157. [DOI] [PubMed] [Google Scholar]
- Patel K., Moore S. E., Dickson G., Rossell R. J., Beverley P. C., Kemshead J. T., Walsh F. S. Neural cell adhesion molecule (NCAM) is the antigen recognized by monoclonal antibodies of similar specificity in small-cell lung carcinoma and neuroblastoma. Int J Cancer. 1989 Oct 15;44(4):573–578. doi: 10.1002/ijc.2910440402. [DOI] [PubMed] [Google Scholar]
- Paulin D., Forest N., Perreau J. Cytoskeletal proteins used as marker of differentiation in mouse terato;carcinoma cells. J Mol Biol. 1980 Nov 25;144(1):95–101. doi: 10.1016/0022-2836(80)90216-8. [DOI] [PubMed] [Google Scholar]
- Radice P. A., Matthews M. J., Ihde D. C., Gazdar A. F., Carney D. N., Bunn P. A., Cohen M. H., Fossieck B. E., Makuch R. W., Minna J. D. The clinical behavior of "mixed" small cell/large cell bronchogenic carcinoma compared to "pure" small cell subtypes. Cancer. 1982 Dec 15;50(12):2894–2902. doi: 10.1002/1097-0142(19821215)50:12<2894::aid-cncr2820501232>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rosengard A. M., Krutzsch H. C., Shearn A., Biggs J. R., Barker E., Margulies I. M., King C. R., Liotta L. A., Steeg P. S. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989 Nov 9;342(6246):177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- Strahler J. R., Lamb B. J., Ungar D. R., Fox D. A., Hanash S. M. Cell cycle progression is associated with distinct patterns of phosphorylation of Op18. Biochem Biophys Res Commun. 1992 May 29;185(1):197–203. doi: 10.1016/s0006-291x(05)80975-1. [DOI] [PubMed] [Google Scholar]
- Tome Y., Hirohashi S., Noguchi M., Matsuno Y., Kishi K., Uei Y., Shimosato Y. Immunocytologic diagnosis of small-cell lung cancer in imprint smears. Acta Cytol. 1991 Sep-Oct;35(5):485–490. [PubMed] [Google Scholar]